Positive Health Online
Your Country

How a Mother’s Immunity may cause Developmental and other Disorders in her Children before and after Birth
listed in immune function, originally published in issue 278 - May 2022
Introduction
It is important that all health carers, those who take responsibility for other peoples’ health, understand the basic principles of immunity because, by far, most forms of ill-health are caused by aberrations in one of more elements of the immune machinery.
The immune machinery comprises many different components. They are basically divided into the adaptive and the innate immune systems. The adaptive system is derived from cells which originate from bone marrow stem cells. It responds to a wide range of infectious agents, toxic and foreign substances that everyone is exposed to on a daily basis. The adaptive immune system may take two weeks or more to respond.
The innate or non-adaptive immune system supports the adaptive immune system’s activity when it has responded to a particular foreign substance, toxin or infectious agent. The innate immune system also provides a degree of protection until the adaptive system has developed a specific immune response.
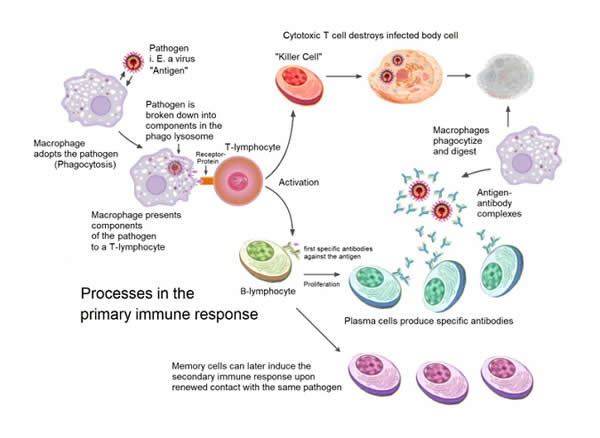
Simplified Overview of the Processes Involved in the Primary Immune Response
Credit: Wikipedia
The adaptive immune system is derived from different types of white cells. One type of adaptive white cell called a cytotoxic T cell can kill cancer cells and cells infected with a virus. Another type of white cell called a B cell can produce a series of soluble proteins called immunoglobulins or antibodies that can bind to and kill infectious invaders such as bacteria, fungi and parasites. These antibodies can also neutralize toxins. Unfortunately, antibodies can also cause the development of very many different forms of ill-health.
There are many ways by which immune aberrations can cause development of a range of different diseases. For example, reduced immunity results in increased susceptibility to many infectious diseases. On the other hand, increased immunological activity can lead to the onset of many inflammatory mediated autoimmune disorders.
There are more than 200 different types of cells in the human body, each with a different biological or neurological function. It is possible that an autoimmune process can target any one of them. In fact thus far, the pathogenesis of well over a hundred diseases has been attributed to autoimmunity. For brevity, and because there are so many ways that immune aberrations can cause disease, this article has been prepared to bring the reader’s attention specifically to the way in which a mother’s isoantibodies or autoantibodies can support the health or cause disease in her children before and after birth.
B cells have the capacity to synthesize a number of different types of antibody. The different types of antibody are referred to as Ig (immunoglobulin) G, IgM, IgA, IgE and IgD. Each type of immunoglobulin molecule has a different function.
On exposure to an infectious agent, IgM antibodies are the first type of antibody to be produced. They are of large size and are found mainly in the blood. Thus, they protect against infectious and foreign agents such as bacteria in the bloodstream. On the other hand, IgG antibodies which are produced later, after subsequent exposure to the same infectious or foreign agent, are much smaller in size and much more abundant than IgM antibodies. Because of their size, IgG antibodies can pass from the bloodstream into interstitial fluids to provide more extensive immunological protection.
There are four different subtypes of IgG referred to as IgG1, IgG2, IgG3 and IgG4. IgG1, IgG3 and IgG4 subtypes can cross the placenta during pregnancy, IgG2 cannot. Thus, antibodies of the IgG subtypes IgG1, IgG3 and IgG4 play an important role in provision of immunological protection for the unborn child and for a few months after birth depending on the specificity the mother’s antibodies. IgG1 and IgG3 subclasses are rich in antibodies against proteins such as the toxins produced by the diphtheria and tetanus bacteria, as well as antibodies against viruses. IgG4 antibodies offer protection against development of allergic disease.
IgG2 antibodies react predominantly against the polysaccharide coating of disease-causing bacteria such as Streptococcus pneumoniae and Haemophilus influenzae. Thus because IgG2 antibodies are not passed on to the child during pregnancy, infection of the foetus by bacteria such as Streptococcus pneumoniae and Haemophilus influenzae can lead to miscarriage and foetal death.
Unfortunately, many diseases can be caused in the child before and after birth if a mother’s IgG antibodies that cross the placenta also contain antibodies that react with the baby’s tissues.
Role of Maternal Isoantibodies
Before and during birth, some of the baby’s blood components such as various blood cells and other proteins pass into the mother’s circulation. If the baby’s cells are genetically or otherwise different to the mother’s, the mother’s immune system may regard them as immunologically foreign and produce IgG antibodies against them. These antibodies can then pass through the placenta and cause harm to the unborn child depending on which cells or proteins have been targeted by the mother’s IgG antibodies.
Expectant Woman
Acknowledgement Credit: Unsplash
One of the best known foetal diseases caused by a mother’s immunity is haemolytic disease of the newborn (HDN). It is usually caused by transfer of maternal IgG Rhesus antibodies across the placenta and into the circulation of a Rhesus positive foetus in the womb.
Maternal Rhesus antibodies are induced following passage of a baby’s Rhesus positive (D+) red cells into the bloodstream of a Rhesus negative (D-) mother during pregnancy and at birth. The Rhesus negative mother’s immune system recognizes the Rhesus D+ antigen on the baby’s red cells as immunologically foreign. The mother’s immune system then makes an antibody against the D antigen on the baby’s red cells. As it takes a while before IgG anti-D antibodies are formed in the mother’s blood, it is always the second or subsequent Rhesus D+ babies that are likely to be affected. Binding of the mother’s anti-D antibody to the baby’s D+ red cells causes destruction of the baby’s red cells, severe anaemia, jaundice and sometimes death. The Rhesus blood group system includes a number of different components including C, E, c and e antigens. These other Rhesus components can also be the cause of a Rhesus based HDN.
There are around 30 different red cell blood group systems. Some of them such as the Kell, Kidd, MNS and Duffy blood group systems for example, can also be a cause of IgG antibody mediated HDN.
HDN can be caused by ABO incompatibility, however, this form of haemolytic disease is not as severe as Rhesus blood group based HDN.
HDN is treated by transfusing the baby with red cells that do not react with the causative antibody found in the mother’s blood. Interestingly, the prospect of development of HDN may be pre-determined by testing for the presence of the mother’s IgG antibodies that react against the father’s red cells.
Other types of baby’s blood cells such as the white cells or leukocytes including lymphocytes and neutrophils (the most abundant type of granulocyte) as well as platelets also pass into the mother’s circulation during pregnancy and at birth. The mother may also produce antibodies to components of these cells if they differ genetically from the mother’s cells. Placental passage of a mother’s IgG human leukocyte antigen (HLA) antibodies into the baby’s circulation may cause a premature birth, particularly with respect to differences in members of the HLA-C lymphocyte system.[1]
Neutrophils are bone marrow derived phagocytic cells that play an important role in engulfment of infective agents such as bacteria, viruses and parasites. They express a number of different genetically determined antigen systems called HNA1, HNA2 and so on, each with a different number of alleles. If any of the HNA antigen systems differ between mother and child, she may produce an IgG type of antibody to the baby’s neutrophils which can cross the placenta and reduce the numbers of neutrophils in the foetus. Reduced numbers of neutrophils (neutropenia) in the foetus pre- and post-delivery may result in miscarriage or serious infection post-partum.[2]
Platelets (thrombocytes) are small cells that play an important role on arresting bleeding. There are around 30 different genetic antigen systems on platelets. They are known as human platelet antigens HPA-1, HPA-2 and so on.
Mothers who are genetically HPA-1b can readily make IgG antibodies to the HPA-1a antigen of the platelets of an HPA-1a positive foetus. Maternal platelet antibodies cross the placenta and destroy the baby’s platelets resulting in excessive bleeding of the child pre- and post-partum. These types of disorders can be treated by transfusion of blood into the baby that is shown not to react with any of the mother’s disease-causing IgG antibodies.[3]
Some subjects, because of their genetic background and environmental exposure, are prone to development of a wide range of antibodies against many different biological molecules including foetal proteins that are required for optimum development in the womb.
In the past, it was postulated that, because maternal IgG antibodies are able to cross the placenta, some childhood disorders may be caused by impairment of some early developmental processes in the womb because of the presence of maternal IgG isoantibodies directed against critical foetal developmental proteins. In recent years, this postulate has been proven. It is now understood that development of autism, autism spectrum disorders and learning difficulties in early childhood is associated with the presence of maternal IgG antibodies against many proteins involved in neurological development in the foetus. Critical foetal development proteins targeted include lactate dehydrogenase A and B, stress-induced phosphoprotein 1, guanine deaminase, collapsin response mediator proteins 1 and 2 and Y-box binding protein. The mother’s anti-brain developmental antibodies gain access to the foetal brain early in pregnancy before the baby’s blood/brain barrier is fully formed.[4]
The Role of Maternal Autoantibodies
All the above pathological conditions are invariably caused by maternal isoantibodies, that is, antibodies that do not react with the mother’s own tissues. Some mothers may have autoantibodies which react with their own tissues and cause a wide range of autoimmune diseases. Such autoantibodies of the IgG type may also cross the placenta during pregnancy and cause similar diseases in the baby.
Mothers who suffer from the autoimmune diseases systemic lupus erythematosus (SLE) or Sjögren’s syndrome often have a wide range of IgG autoantibodies including some called anti-Ro (SSA) and La (SSB). These autoantibodies, particularly anti-Ro can cause irreversible heart damage in the child. This irreversible cardiac damage is related to the expression of Ro and La proteins in the baby’s developing heart tissue during the 18th to 24th weeks of gestation. Under these circumstances, the proper development of cardiac tissue in early pregnancy is impaired. This may lead to development of heart block at birth and increased susceptibility to heart problems in later life.
Many other neurological disorders including dyslexia, learning difficulties and other neurological problems have been found in the offspring of mothers suffering from SLE.[5] These findings are consistent with the notion that many neurological disorders may be pre-determined in the womb by exposure to a range of maternal iso- and autoantibodies pre- and post-partum.
Implications for Surrogacy
Ideally, potential surrogates should lack the antibodies as described above. Tests are available in some Clinical Immunology laboratories for detection of most of these harmful IgG antibodies.
A fully comprehensive immunopathology course dealing with all aspects of immunity in health and disease which is suitable for all health practitioners and students of the health sciences has been prepared by the author. It is now available from the Complementary Medical Association (The CMA). This groundbreaking course can be found at: cmacourses.org/immunopathology
References
- https://onlinelibrary.wiley.com/doi/10.1111/aji.12797
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6489100/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3651544/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5373490/
- https://lupusnewstoday.com/2017/06/29/study-indicates-children-of-mothers-with-lupus-are-more-prone-to-developmental-disorders/
Comments:
-
No Article Comments available