Positive Health Online
Your Country

Mushroom Nutrition as a Disease Modifying Therapy for Neurodegenerative Conditions? Part IV-Neurogenic Reserve
listed in neurological and neurodegenerative, originally published in issue 271 - June 2021
Mushroom Nutrition as a Disease Modifying Therapy for Neurodegenerative Conditions? Part IV-Neurogenic Reserve
by William Ahern
To date the development of a pharmaceutical solution to Alzheimer’s Disease (AD) to reduce the increase of both Tau and β-Amyloid protein production has been disappointing.
When faced with patients with early signs of dementia, should clinicians consider the use of mushroom nutrition to:
- Reduce neuroinflammation (Short Takes-Issue 263-June 2020);
- Combat viral infections that could be responsible for triggering dementia (Short Takes-Issue 265-September 2020);
- Establish a healthy microbiota in dementia diagnosed patients (Short Takes-Issue 267-January 2021); and
- Increase neurogenic reserve via mushroom nutrition?
Brain cognitive reserve reflects the brain capacity to preserve normal functions.[1] As outlined in Table I, there are several factors that modulate cognitive reserve:
The impact of the aforementioned factors can translate into physical changes in brain morphology as outlined in Table II.
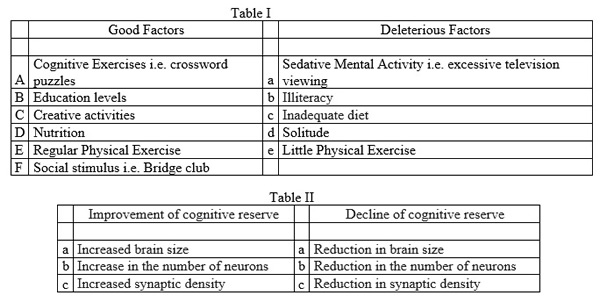
Neurogenesis is the process by which new neurons are formed in the brain. Neurogenesis is crucial when an embryo is developing, but also continues in certain brain regions after birth and throughout our lifespan. The mature brain has many specialised areas of function, and neurons that differ in structure and connections.
The hippocampus, for example, which is a brain region that plays an important role in memory and spatial navigation, alone has at least 27 different types of neurons.
Adult hippocampal neurogenesis is a process that describes the generation of new functional DGCs from adult neural stem cells through the amplification of intermediate progenitors and neuroblasts, as well as the integration of these new neurons into the existing neural circuits. The hippocampal neuron growth stages are outlined below.
The hippocampal neuron growth stages are outlined in: Boldrini M, Fulmore CA, Tartt AN, et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell. 2018;22(4):589-599.e5. doi:10.1016/j.stem.2018.03.015
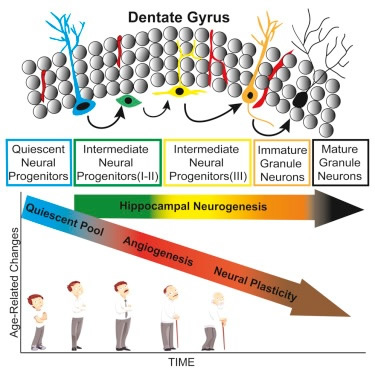
Hippocampal neurons are derived from stem cells, then quiescent neural progenitors, to Intermediate Neural Progenitors (I -II), then to Intermedial Neural Progenitors (III) and then to Immature Granule Neurons and then Mature Granule Neurons.
Scheme of the current view of lineage progression and fate decisions during adult hippocampal neurogenesis. A, astrocyte; GC, granule cell; NSC, neural stem cell. Urbach A, Witte OW. (2019). Divide or Commit – Revisiting the Role of Cell Cycle Regulators in Adult Hippocampal Neurogenesis. Front. Cell Dev. Biol., 24 April 2019 | https://doi.org/10.3389/fcell.2019.00055
Importance to Maintain Healthy Hippocampal Neurogenesis.
In Alzheimer´s disease and other forms of dementia, there is a malfunction of the growth stages of hippocampal neurons or an unexplained severe deletion of these neurons or inability of these neurons to establish an integration within the existing circuitry of hippocampal function.
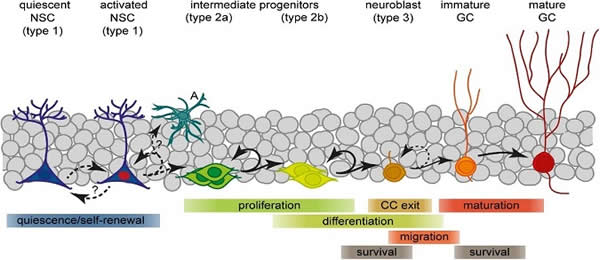
As part of this process the newly generated granule cells then extend axons or dendritic branches to integrate themselves in the pre-existing network of cells that compose the hippocampal. Again, the integration relies on newly formed dendritic branches of pre-matured neurons that reach the dentate gyrus (DG) molecular layer (ML).
From granule cell layer (GCL) and upon reaching the molecular layer (ML) layer these dendritic branches reach out to entorhinal cortex (EC) and lateral (LPP) and medial (MPP) path, forming novel synaptic connections.
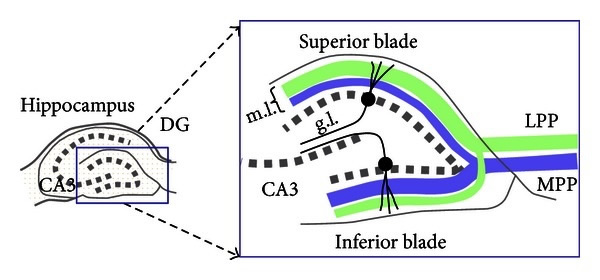
These cells can then project their axons to the Cornu Ammonis 3 (CA3), further establishing the hippocampal circuitry; hence maintaining or improving dendritic complexity is key to hippocampal function.
When there is a malfunction in any of the processes, then a subsequent malfunction can be expected in either the quiescent pool (stem cell generator), angiogenesis or neural plasticity leading to a loss of memory.
Therefore, one of the key objectives in the treatment of Alzheimer´s or dementia should be to maintain the healthy functioning of the hippocampal neurogenesis.
Importance of Wnt/ β-catenin Signalling
The Wnt are secreted glycoproteins acting through transduction pathways signalling cascade and ligands, controlling carcinogenesis, embryonic development and tissue regeneration. They are dual function proteins, involved in regulation and coordination of cell–cell adhesion and gene transcription, playing an important role in neurogenesis by enhancing the maturation of the newly generated dendritic network.
The proteins that are often mutated in neurodegenerative diseases have the ability to regulate β-catenin levels, factor in adult neurogenesis, synaptic plasticity, and the function of thalamic neurons.
Study Design
In 2018, researchers at the Institute for Pharmacology and Experimental Therapeutics (IBILI), Faculty of Medicine at the University of Coimbra Portugal and in the Center for Neuroscience and Cell Biology at the University of Coimbra Portugal led by Professor Frederico C. Pereira and Professor Cristina Ana Rego performed the following study on mice to evaluate the impact of mushroom nutrition on hippocampal neurogenic reserve.
Using Coriolus versicolor (CV) biomass supplementation, the researchers took two groups of 2.5-month-old mice were divided into one group taking 200 mg/kg every day for 2.5 months while the control group took saline.
After 2.5 months the mice were sacrificed, and the following parameters were measured and compared between the two groups.
Results
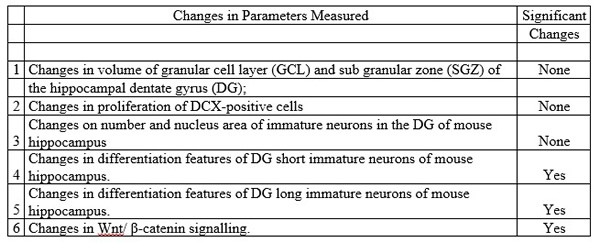
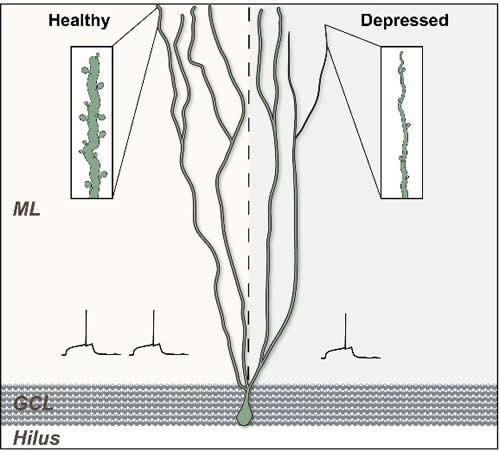
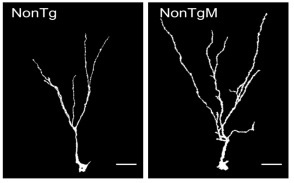
While there was no changes in the volume or proliferation in newly generated neurons, it was determined that mice treated with Coriolus versicolor biomass supplementation had a significant increase in the complexity of the long and short immature neurons (increase in dendritic complexity).
Of additional interest there was the fact that mice treated with Coriolus versicolor biomass had increased
a. Wnt/ β-catenin signalling which plays an important role in the differentiation of neuroblasts into mature neurons; and
b. increases of both cytoplasmic and nuclear levels of β-catenin in immature neurons from hippocampal DG, suggesting that this protein may be a key molecule responsible for the increase in dendritic complicity in these cells.
Conclusion
The Portuguese researchers demonstrated that supplementation with Coriolus versicolor biomass in mice for 2.5 months promotes:
a. the increase dendritic arborization of newly generated neurons (short and long); (increase in dendritic complexity) [2] and
b. the increase in the levels of β-catenin in the nucleus and cytoplasm of hippocampal newly generated neurons.
Taken together this indicates that CV promotes hippocampal neurogenic reserve that may translate into enhanced cognitive reserve.
Further research is required to reconfirm these results.
Further Information
The Coriolus versicolor used in this study was supplied by Mycology Research Laboratories Ltd. (Luton, UK). ( www.mycologyresearch.com ).
References:
- University of Queens Land-Queens Land Brain Institute-home page https://qbi.uq.edu.au/brain-basics/brain-physiology/what-neurogenesis
- Ferreiro E et al. Coriolus versicolor biomass increases dendritic arborization of newly-generated neurons in mouse hippocampal dentate gyrus. Oncotarget, 2018, Vol .9 (no 68) pp:32929-32942. https://pubmed.ncbi.nlm.nih.gov/30250640/
Comments:
-
No Article Comments available