Positive Health Online
Your Country

Does Inflammation Cause Coronary Atherosclerosis?
by Carlos ETB Monteiro(more info)
listed in heart, originally published in issue 268 - February 2021
Authors
Carlos ETB Monteiro - Independent Researcher and Scientist President, Infarct Combat Project (www.infarctcombat.org ). Fellow, American Institute of Stress
Paul J. Rosch, MD - Clinical Professor of Medicine and Psychiatry New York Medical College; Chairman, The American Institute of Stress (www.stress.org ). Obituary Tribute to Dr Paul J Rosch MD MA FACP: June 30, 1927 - February 26, 2020 published in Positive Health PH Online Letters to the Editor Issue 262 May 2020

Available on Amazon.co.uk and Amazon.com
https://www.amazon.co.uk/Autonomic-Dysfunction-Acidosis-Multiple-Diseases/dp/B086PT97PJ
Acknowledgement Citation
The present article first appeared in the Book Autonomic Dysfunction + Lactic Acidosis = Multiple Diseases, 2020, by Carlos ETB Monteiro, at https://amzn.to/2Eyagy0 Available on Amazon.co.uk and Amazon.com.
Abstract
It has been proposed that inflammation, as defined by an elevated hs-CRP [high-sensitivity C-reactive protein], causes coronary atherosclerosis and contributes to numerous other diseases. However, this inflammation is a response to injury to the endothelial layer of the coronary arteries, which can be due to infections and other irritants. In addition, if inflammation caused coronary heart disease, then why did powerful nonsteroidal anti-inflammatory drugs increase heart attacks in low risk patients? In addition, this inflammatory process cannot be felt or seen, in contrast to the heat, swelling, pain and redness of inflammation as it was defined by Celsius, so perhaps it should be called something else to avoid confusion. This presentation will discuss various influences that affect inflammation, such as the pivotal role of sympathetic nervous system stimulation and humoral influences that also regulate heart rate and other vital functions. In that regard, studies show that inflammation is associated with an increase in heart rate as well as reduced heart rate variability, a powerful predictor of risk for coronary heart disease and sudden cardiac death. Inflammation is also associated with an increased acid environment due to metabolic acidosis, which affects the function of monocytes and macrophages that modulate immune system responses. Stimulation of the sympathetic nervous system also promotes glycolysis and increases lactic acid and lactate production that further lower pH. In contrast, stimulation of the vagus nerve and parasympathetic activities have anti-inflammatory effects that provide significant benefits in patients with rheumatoid arthritis, depression and other diseases that are unresponsive to conventional therapies. We will discuss the role of canakinumab in heart disease patients with an elevated hs-CRP [high-sensitivity C-reactive protein] signifying increased inflammation, as revealed in the CANTOS trial. Canakinumab is a monoclonal antibody that lowers hs-CRP and inhibits interleukin-1β, another marker of inflammation. Although both were lowered, the treated group had more deaths from infection and the FDA rejected the request for the treatment of heart disease. We will also discuss Omega-3 fatty acids, the surprising anti-inflammatory effects of cardiac glycosides, and the role of lactate and acidosis.
Atherosclerosis And Inflammation
There have been increasing claims that coronary atherosclerosis is due to a silent “low-grade chronic inflammation “. This is associated with an increase in hs-CRP (high sensitivity C-reactive protein), which measures levels much lower than the traditional CRP test used for more than 8 decades to assess the degree of acute inflammation. Over 2000 years ago, Celsus defined inflammation as heat, swelling, redness and pain. But all of these can be seen or felt, whereas this subtle chronic inflammation produces no signs or symptoms. Nor does it respond to anti-inflammatory drugs or antibiotics. It has also been proposed that hs-CRP actually causes inflammation and coronary atherosclerosis, rather than being a mere marker like high LDL and low HDL. The JUPITER rosuvastatin trial contradicted this, since although LDL-C was reduced by 50%, the largest drop in any statin trial, and hs-CRP was lowered by 37%, there were more fatal heart attacks in the statin treatment group. Moreover, as noted above, reducing inflammation does not reduce heart disease. Vioxx, a powerful non-steroidal anti-inflammatory drug, was taken off the market because it caused heart attacks, and other anti-inflammatory drugs have similar effects.[1]
Rudolph Virchow, who first noted the presence of cholesterol in atheroma, described what would later be called atherosclerosis as “endarteritis deformans”.[2] The “itis” signified inflammation, because he did not believe that atherosclerosis was due to the deposition of cholesterol, since this came later.
Van Haller used the Greek word "'atheroma" to refer to a gruel- like material in 1775, but "atherosclerosis" did not appear until 1904, when Marchand coined it to indicate a hardening of this material in the walls of arteries. A few years earlier, Lobstein had introduced arteriosclerosis to depict a calcification of these arterial lesions as they aged.
Vascular calcifications can be classified into 2 separate types depending on whether they are located within the intimal or medial layer. Medial arterial calcification primarily affects the legs, and is prevalent in patients with peripheral vascular disease. Intimal calcification predominates in coronary vessels. While both types can be found in the carotid arteries and they share some features, they have different causes and consequences.
Although atherosclerosis and arteriosclerosis are relatively recent terms, these disorders are not new. CT analyses of Egyptian mummies and other ancient cultures where corpses were well preserved due to very dry or cold conditions, such as the Peruvian Incas, the Aleutian Island Unangans and the Ancestral Puebloans of southwest America, reveal that they were not uncommon 3500 to 4000 years ago, especially in the elderly and elite. Atherosclerosis was regarded as definite if a calcified plaque was seen in the wall of an artery, and probable if it was seen along the expected course of an artery. Most of this calcification occurs in large vessels like the aorta, iliac and carotid arteries. It is primarily medial arteriosclerosis since the deposits are found in the muscular middle layer of the arterial wall, and is often called Mönckeberg's sclerosis, after Johann Georg Mönckeberg, who first described it in 1903. Although it differs from coronary atherosclerosis, it is likely that was also prevalent in individuals who lived longer.
The Autonomic Nervous System And Inflammation
The involuntary nervous system maintains stability in the body whenever homeostasis is threatened via its complementary but antagonistic sympathetic and parasympathetic constituents. The sympathetic nervous system and its neurotransmitters are stimulated during acute stress, which results in an increase in heart rate and blood pressure and a host of other activities throughout the body to facilitate “fight or flight”.
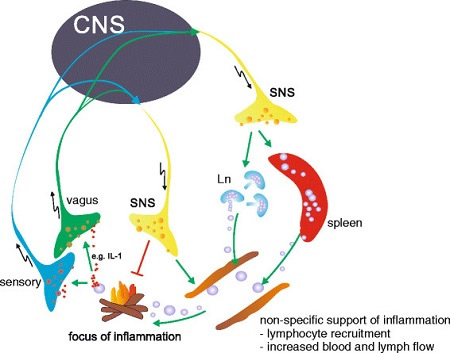
Sympathetic Nervous System Responses To Inflammation
As follows, local inflammation is detected by vagal and sensory nerve fibers that have receptors for inflammatory mediators like interleukin and a signal sent to the brain’s central nervous system (CNS) that leads to activation of the sympathetic nervous system (SNS) and the release of neurotransmitters like noradrenaline at the site, which has an anti-inflammatory effect that is a transient response to a local threat
There is a marked increase in neutrophils, white cells that inactivate bacteria and their toxic products and promote tissue repair. Stimulation of the hypothalamic-pituitary-adrenal axis causes a rise in the secretion of cortisol that initially reduces inflammation, but this only lasts for a day or two. This contrasts with chronic systemic inflammation, which can evoke a cascade of non-specific responses such as recruitment of lymphocytes, white cells that can recognize and respond to antigens by producing antibodies. There is also increased lymph and blood flow to the affected areas. When inflammation persists and becomes chronic, the sympathetic nervous system and hypothalamic-pituitary-adrenal responses continue to be activated, but the anti-inflammatory effects of cortisol and other glucocorticoids dwindle, which ultimately results in tissue damage and organ dysfunction. Macrophages, fibroblasts, mast cells, monocytes and other immune system cells and cytokines can also contribute to this.
The parasympathetic nervous system also modulates chronic inflammation by vagal stimulation through the cholinergic anti-inflammatory pathway.
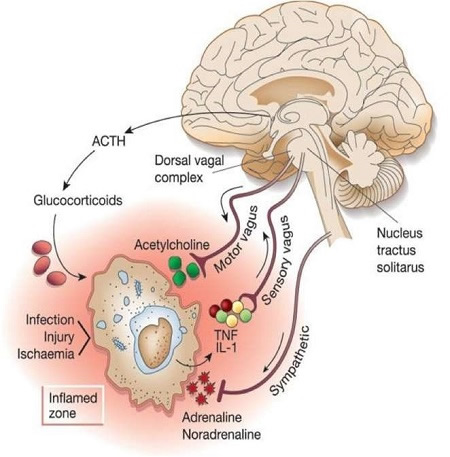
Autonomic Nervous System Responses To Inflammation
As follows, inflammatory products and debris produced by damaged tissues activates afferent signals that are transmitted to the nucleus tractus solitarus. Subsequent stimulation of vagus efferent activity Inhibits cytokine synthesis via the anti-Inflammatory cholinergic pathway. Information can also be relayed to the hypothalamus as well as the dorsal vagal complex to stimulate the production of ACTH by the anterior pituitary. This results in an increased secretion of glucocorticoid hormones like cortisol that also decrease inflammation.
These observations have led to the development of new approaches to treating inflammation, such as modulating vagus nerve activity, or targeting specific components of this complex pathway. Meditation, biofeedback and other stress reduction measures, as well as hypnosis or acupuncture also have the potential to modulate vagal output. A variety of non-steroidal anti-inflammatory and psychoactive drugs could be designed to stimulate macrophage cholinergic receptors in the periphery or to increase vagal output comparable to a pharmacological vagus nerve stimulator. Vagal nerve stimulation has resulted in spectacular results in rheumatoid arthritis that is resistant to all other therapies, and has none of their adverse side effects or addictive tendencies. It may also be effective in Crohn’s disease and other inflammatory bowel disorders, as well as drug resistant depression. This non-invasive treatment is administered by the patient at home, and the dosage, frequency and duration of stimulation can easily be changed as needed.
Support for the protective effect of the parasympathetic nervous system in preventing or reducing inflammation and atherosclerosis can be found in numerous articles, and excerpts or synopses from some, are appended below:
- 2007 - “Based on converging evidence, we propose a neuroimmunomodulation approach to atherogenesis. In this model, the vagus nerve "informs" the brain about coronary artery disease related cytokines; in turn, activation of the vagus (via vagus nerve stimulation, vagomimetic drugs or relaxation) induces an anti-inflammatory response that can slow down the chronic process of atherogenesis.”[3]
- 2011- “It is also likely that in the future, the currently available treatment regimens for coronary heart disease, cardiac arrhythmias and atherosclerosis could be combined with vagus nerve stimulation and nicotinic acetylcholine receptor α7 subunit agonists.”[4]
- 2012 - “The inflammatory reflex mediated by the vagus nerve has been successfully exploited therapeutically in preclinical models of diseases with aetiologies characterized by excessive inflammatory responses”, and that “Insufficient efferent vagus nerve cholinergic output might have a causative role in the dysfunctional immune and metabolic regulation observed in obesity, as selective activation of the efferent cholinergic arm of the inflammatory reflex attenuates both inflammation and metabolic derangements.”[5]
- 2014 – “Central cholinergic activation of a vagus nerve–to spleen circuit controls alleviates intestinal inflammation.”[6]
- 2016 - Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis.[7]
Regulation Of Inflammation by the Sympathetic Nervous System and Acidosis
- 2012 –“Extracellular acidosis downregulates most of the hemostatic platelet functions and promotes those involved in amplifying the neutrophil-mediated inflammatory response.” (Platelet aggregation at sites of vascular injury is considered essential for hemostasis and arterial thrombosis.)[8]
- 2012 – “The discovery that cholinergic neurons inhibit acute inflammation has qualitatively expanded our understanding of how the nervous system modulates immune responses. The nervous system reflexively regulates the inflammatory response in real time, just as it controls heart rate and other vital functions.”[9]
- 2013 – Data is provided suggesting that an acidic environment represents a novel endogenous danger signal alerting the innate immunity. “Low pH may thus contribute to inflammation in acidosis-associated pathologies, such as atherosclerosis and post-ischemic inflammatory responses.”[10]
- 2014 - “Over the past decades evidence has accumulated clearly demonstrating a pivotal role for the sympathetic nervous system (SNS) and its neurotransmitters in regulating inflammation.” The authors concluded “However, if a ‘chronic inflammatory configuration’ persists, as in autoimmunity, the effects are detrimental because of the persistently increased SNS activity, HPA activity, and the resultant chronic catabolic state. This leads to known comorbidities in chronic inflammatory disease, like cachexia, high blood pressure, insulin resistance, and increased cardiovascular mortality. The challenge is now to translate this conceptual knowledge into clinical benefit.”[11]
- 2016 – Study demonstrating that moderate extracellular acidosis, which is a common finding in different pathological conditions such as inflammation, ischemia or in solid growing tumors, affects the functional behavior of monocytes and macrophages, and can therefore modulate the immune response.[12]
Canakinumab Anti-inflammatory Therapy and Atherosclerosis
One of the highlights of the August 2017 European Society of Cardiology Congress was the landmark CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study) showing that decreasing inflammation, even in the absence of any lipid lowering, significantly reduced recurrent cardiovascular events in patients with a history of myocardial infarction and an hs C-reactive protein of 2 mg or more per liter.[13]
It also reduced cancer incidence and mortality. Canakinumab is currently indicated for the treatment of interleukin-1ß associated inflammatory diseases, and this study allegedly provided strong support for the belief that inflammation caused recurrent coronary events because it increased atherosclerosis. In fact, the title of the paper that was published in the September 21, 2017 New England Journal of Medicine was ‘Anti-inflammatory Therapy with Canakinumab for Atherosclerotic Disease.”[14]
However, the reduction in recurrent cardiovascular event rates showed only a 2% absolute risk reduction study over median follow-up of 3.7 years, Canakinumab was associated with a higher incidence of fatal infections compared to placebo and there was no significant difference in all-cause mortality. In addition, the $16,000-per-dose price tag meant that treatment would cost approximately $200,000 per year, and many doctors also expressed concerns about the future of this drug based on the study results. Some argued that the same results might have been obtained with existing and much less expensive drugs.[15] In October 2018 the FDA declined to approve Canakinumab for cardiovascular risk reduction based on the CANTOS results.[16]
Not mentioned was the fact that numerous studies have shown that C-Reactive Protein is elevated with chronic stress as well inflammation. Danish researchers also found that higher hs- CRP blood levels were associated with a greater risk of psychological stress and especially clinical depression. As the lead author noted, “Irrespective of other factors, we found that basically healthy people with hs-CRP levels above 3 milligrams per liter had a two- to threefold increased risk of depression. Dampening inflammation may be one way of treating depression.” It is not clear what explains this association, but the authors suggest that elevated CRP levels may indicate elevated levels of certain cytokines that can increase feelings of stress, or that depression itself may lead to increased inflammation.[17]
Myocardial ischemia provoked in the laboratory during acute mental stress in patients with stable coronary artery disease predicts subsequent clinical events, much like exercise induced ischemia with treadmill stress tests, but the mechanisms responsible for this are different. While sympathetic nervous system activation may play a role, little is known about how mental stress increases risk for coronary events. Since an elevated hs-C-reactive protein is also a risk marker for future coronary events in patients with heart disease, perhaps increased inflammation was responsible. To evaluate this, 83 patients with stable heart disease underwent simultaneous single-photon emission computed tomography (SPECT) and transthoracic echocardiography (TTE) at rest, and during laboratory induced mental stress. Serum hs-CRP levels were measured before and 24 hours after mental stress. Of the 83 patients, 30 (36%) showed ischemic changes due to mental stress. There was no difference in gender, sex, BMI, histories of diabetes, hypertension, smoking, lipid profile, medications used (including statins, β-blockers, ACE inhibitors, and aspirin), or hemodynamic responses during mental stress between this group and those who had no evidence of ischemia. However, they did show a greater increase in hs-CRP, and each 1 mg/L increase in this level was associated with a 20% higher risk of mental stress induced ischemia.[18] However, this does not prove that either hs-CRP or inflammation cause heart disease, since association never proves causation. Other studies have found that emotional stress can provoke ischemia in 30%–50% of patients with chronic, stable coronary disease. [19,20]
Chronic stress, especially job stress,[21] socioeconomic status,[22] as well as social and personality factors have also been associated with increased risk of coronary heart disease as well as atherosclerotic progression.[23] Acute stress, whether provoked by national emergencies[24,25] or severe anger[26-29] have been associated with triggering cardiac events and heart failure. [30] While mental stress–induced ischemic episodes are good indicators of 5-year rates of cardiac events[31] stress-induced ischemia without angina occurs much more frequently than appreciated.[32,33] There is also evidence that acute stress events such as public speaking and anger-provoking situations can disrupt cardiac electrical signaling and lead to arrhythmias and other acute cardiac events, including myocardial infarction.[34]
Cardiac Glycosides, Omega-3 Fatty Acids and Acidosis
The anti-inflammatory and beneficial effects of digoxin and other cardiac glycosides have been known for decades.[35-42] A 2009 study concluded “Digitoxin elicits anti-inflammatory and vasoprotective properties. These observations indicate a potential therapeutic application of digitoxin in the treatment of cardiovascular diseases, such as atherosclerosis.”[36] Another review that discussed this and other cardiac glycosides and their mechanisms of action by providing “an overview of the in vivo and in vitro actions of cardiac glycosides on inflammatory processes and of the signaling mechanisms responsible for these effects: cardiac glycosides have been found to decrease inflammatory responses in different animal models of acute and chronic inflammation. Regarding the underlying mechanisms most research has focused on leukocytes. In these cells, cardiac glycosides primarily inhibit cell proliferation and the secretion of proinflammatory cytokines”.[35] It is not generally appreciated that glycosides like digoxin,[43] digitoxin,[44] and ouabain [45] inhibit the sympathetic nervous system when administered in low dosages. A study done over 50 years ago showed that they inhibited epinephrine induced glycolysis and glycogenesis in skeletal muscle.[46] A more recent one demonstrated that they also inhibited lactate production and glycolysis in lung cancer cells, which require glucose, and increase the cytotoxicity of platinum compounds that are frequently used to treat lung cancer.[47]Other drugs or factors might provide similar benefits. Omega-3 fatty acids inhibit atherosclerosis without lowering cholesterol or triglycerides,[48]and some, like docosahexaenoic acid, can improve heart rate variability and baroreflex sensitivity via effects on the autonomic nervous system.[49] However, their ability to lower elevated blood lactic acid levels.[50,51] may be even more important with respect to inhibiting coronary atherosclerosis.
Lactic Acidosis, Inflammation and Coronary Atherosclerosis
It has been known since 1934 that “Acidity of the environment is increased in inflammatory sites.[52]Over a half century ago, it was proposed that osteoporosis was due to an “acid ash” die, and that this was buffered by bone minerals.[53]A review of this issue in 2018 confirmed that that even subtle chronic acidosis can cause appreciable bone loss if prolonged.[54] It was demonstrated that lactic acidosis is associated with coronary disease and atherosclerosis, and explained why it increases coronary artery calcification,[55]. This is consistent with the acidity theory of atherosclerosis [56,57]that also explains how stress can cause coronary atherosclerosis.[58]It proposes autonomic dysfunction as a precursor, and particularly stimulation of the sympathetic nervous system. This increases the secretion of adrenaline and noradrenaline, which accelerates glycolysis, and results in higher concentrations of lactic acid and lactate in blood, other body fluids and tissues. Conversely, stimulation of the parasympathetic system has anti-inflammatory effects. Other factors such as age, genes, gender, lifestyle and various drugs can also influence the positive or negative effects of autonomic activities.
Association never proves causation, and as others have warned, “Correlation implies association, but not causation. Conversely, causation implies association, but not correlation.[59] Association should not be confused with causality unless it is clear that A always causes B and there is no other cause. Tuberculosis is one example, since it can only be caused by the tubercle bacillus, so this is not a theory but a fact. However, A and B could also be associated but not interdependent, because they are both caused by something else. An elevated cholesterol and hypertension are associated with coronary atherosclerosis, but are not always present, since this is a multifaceted disorder that can have many associated risk markers. Unlike tuberculosis, there is no vera causa or solitary true cause so that all other explanations are merely theories. As the famous philosopher Karl Popper stated, “A medical hypothesis cannot be proved, but it can be falsified. If it cannot be falsified, it is not a scientific hypothesis.”[60] The prevailing hypothesis that cholesterol causes coronary atherosclerosis has been refuted so many times, that it is no longer tenable. It has been replaced by a process that has been labelled inflammation, but bears no resemblance to its definition by Celsus, since it has no signs or symptoms. It can only be detected by elevations of hs-CRP, certain interleukins or other risk markers for inflammation, although these are not always consistent or correlative. This is hardly a new concept, since. as previously indicated, the renowned pathologist Rudolph Virchow, who first demonstrated the presence of cholesterol in atheroma, described atherosclerosis as endarteritis chronica deformans sive nodosa (chronic arterial inflammation with a deforming or knotty appearance), since this is what he saw under the microscope.[2] The suffix “itis” signified that it resembled or was reminiscent of inflammation, but he was very careful to avoid calling it inflammation, since it had none of the tumor, rubor, calor or dolor (swelling, redness, heat, pain) components that Celsus listed, to which Virchow added functio laesa (loss of function.) What Virchow wrote was:
We cannot help regarding the process as one which has arisen out of irritation of the parts stimulating them to new, formative actions; so far therefore it comes under our ideas of inflammation, or at least of those processes which are extremely nearly allied to inflammation.….We can distinguish a stage of irritation preceding the fatty metamorphosis, comparable to the stage of swelling, cloudiness, and enlargement which we see in other inflamed parts.[2]
In other words, inflammation was a response to injury of the “inner arterial coat” (endothelial layer) by some irritant, and the “so-called atheromatous degeneration” (cholesterol deposits) came later. Calling this asymptomatic prophlogistic disorder inflammation is confusing, and hopefully, future advances will lead to a more meaningful definition. As the Nobel Laureate Richard Feynman said, “I learned a long time ago the difference between knowing something and the name of something."
Virchow is still cited to support claims that inflammation rather than cholesterol causes atherosclerosis. Carl von Rokitansky, an eminent contemporary Austrian pathologist who also described inflammatory cells in atheroma, rejected inflammation as the cause of atherosclerosis, and proposed a thrombogenic origin due to the deposition of fibrinogen and other debris from repeated clot formation.[61]
It is interesting to notice that Hans Selye, in 1958, has shown experimentally how stress, combined with some agents, may induce myocardial necrosis where the coronary arteries are perfectly normal. He also said in his paper: “It is noteworthy, however, that, under these circumstances, not only cardiac infarction but organic obstruction of the coronary vessels can regularly be produced by humoral means.”[62]
There are several hundred risk factors for coronary heart disease and atherosclerosis, but the vast majority are simply risk markers that show some statistical association but have no causal relationship.
Coronary heart disease is a multifactorial disorder that can have many causes, some of which, like stress, homocysteine, infections, and free radical damage may be interrelated. Numerous contributing factors that influence susceptibility range from family history, age, genetics, gender, diabetes, hypertension and smoking, to sex hormones, obesity, physical activity, and alcohol consumption. It would be inane to believe that levels of CRP, interleukins or other inflammation markers can provide an accurate assessment of all the varied negative and positive activities of these diverse agencies.
It would be equally foolish to assume that lowering CRP will safely and effectively reduce coronary mortality in healthy people. Treating an elevated CRP would simply repeat the same mistake that is still being made with LDL. As Albert Einstein warned, "Not everything that counts can be counted, and not everything that can be counted counts." The first part of this statement applies to CRP and LDL, which are easy to measure, but have no causal relationships. Association never proves causation. The second part pertains to our inability to define, much less measure, something that we call "inflammation", but may include several different processes that have yet to be elucidated.
We have tried to explain how acidosis, and especially lactate and lactic acidosis can contribute to inflammation and atherosclerosis. We also agree with Virchow’s contention that a process resembling inflammation represents a response to endothelial irritation or injury. In addition, the latter is not due to lipid deposits, since these occurred subsequent to the “swelling and cloudiness” Virchow had observed.
References
- Rosch PJ, Ravnskov U. Why The Lipid Hypothesis of Coronary Heart Disease is Fallacious And Dangerous. 113-136 in PJ Rosch ed. “Fat and Cholesterol Don’t Cause Heart Attacks And Statins Are not the Solution”. 2016; Columbus Publishing Ltd, U.K. at https://www.amazon.com/Cholesterol-Cause-Attacks-Statins-Solution/dp/190779753X
- Virchow R. “Cellular Pathology”: as based upon Physiological and Pathological Histology: Twenty lectures delivered in the Pathology Institute of Berlin, 1856. Published in 1858 at https://www.biodiversitylibrary.org/item/194064#page/64/mode/1up
- Gidron Y, Kupper N, Kwaijtaal M, et al, Vagus-brain communication in atherosclerosis-related inflammation: a neuroimmunomodulation perspective of CAD. Atherosclerosis. 2007 Dec;195(2):e1-9 at https://www.ncbi.nlm.nih.gov/pubmed/17101139
- Undurti N Das. Vagal nerve stimulation in prevention and management of coronary heart disease. World J Cardiol. 2011 Apr 26; 3(4): 105–110 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3082733/
- Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat Rev Endocrinol. 2012 Dec; 8(12): 743–754 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4082307/
- Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve - to spleen circuit alleviates experimental colitis. Mucosal Immunol. 2014 Mar; 7(2): 335–347 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3859808/
- Koopman FA, Sangeeta S, Miljko S et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. PNAS, 2016: vol. 113; no. 29 at http://www.pnas.org/content/113/29/8284.long
- Etulain J, Negrotto S, Carestia A et al. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. Thromb Haemost. 2012 Jan;107(1):99-110 at https://www.ncbi.nlm.nih.gov/pubmed/22159527
- Tracey, K. J. The inflammatory reflex. Nature, 2012; 420(6917): 853–859 at https://www.nature.com/articles/nature01321
- Rajamäki K, Nordström T, Nurmi K et al. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. Journal of Biological Chemistry, Manuscript M112.426254, March 25, 2013 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3650379/
- Pongratz G and Straub RH. The Sympathetic Nervous Response in Inflammation. Arthritis Res Ther.16(504) 2014 at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4396833/
- Riemann A, Wußling H, Loppnow H et al. Acidosis differently modulates the inflammatory program in monocytes and macrophages. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 2016 V1862, Issue 1, Pages 72-81 at https://www.sciencedirect.com/science/article/pii/S0925443915003178?via%3Dihub
- European Society of Cardiology Congress. CANTOS results show anti-inflammatory therapy lowers future cardiovascular events, reduces cancer incidence and mortality. 28 Aug 2017
- Ridker PM, Everett BM, Thuren T et al. Anti-inflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377:1119-1131 at https://www.nejm.org/doi/full/10.1056/NEJMoa1707914#t=article
- Husten L. CardioBrief. Experts Caution on CANTOS and Canakinumab's Future. MedPage Today, August 27, 2017 at https://www.medpagetoday.com/cardiology/cardiobrief/67534
- Nicole Lou. FDA Rejects Canakinumab in CVD Prevention CANTOS data not enough to expand indication. October 19, 2018 at https://www.medpagetoday.com/cardiology/prevention/75811
- Wium-Andersen MK, Ørsted DD, Nielsen SF. et al. Elevated C-Reactive Protein Levels, Psychological Distress, and Depression in 73,131 Individuals. JAMA Psychiatry. 2013;70(2):176-184 at https://jamanetwork.com/journals/jamapsychiatry/fullarticle/1485898
- Shah R, Burg MM, Vashist A et al. C-Reactive Protein and Vulnerability to Mental Stress-Induced Myocardial Ischemia. Mol Med. 2006; Nov-Dec; 12(11-12): 269–274 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1829194/
- Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22:440–8 at https://www.ncbi.nlm.nih.gov/pubmed/8335813
- Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–12 at https://www.ncbi.nlm.nih.gov/pubmed/3352695
- Everson SA, Lynch JW, Chesney MA, et al. Interaction of workplace demands and cardiovascular reactivity in progression of carotid atherosclerosis: population-based study. 1997;314:553–8 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2126071/
- Hemingway H, Shipley M, Macfarlane P, Marmot M. Impact of socioeconomic status on coronary mortality in people with symptoms, electrocardiographic abnormalities, both or neither: the original Whitehall study 25 year follow up.J Epidemiol Comm Health. 2000;54:510–6 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1731713/
- Whiteman MC, Deary IJ, Fowkes FG. Personality and social predictors of atherosclerotic progression: Edinburgh Artery Study.Psychosom Med. 2000;62:703–14 at https://www.ncbi.nlm.nih.gov/pubmed/11020101
- Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334:413–9 at https://www.ncbi.nlm.nih.gov/pubmed/8552142
- Meisel SR, Kutz I, Dayan KI, et al. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet. 1991;338:660–1 at https://www.ncbi.nlm.nih.gov/pubmed/1679475
- Krantz DS, Sheps DS, Carney RM, Natelson BH. Effects of mental stress in patients with coronary artery disease: evidence and clinical implications. JAMA. 2000;283:1800–2 at https://jamanetwork.com/journals/jama/article-abstract/192564
- Mittleman MA, Maclure M, Sherwood JB, et al. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators . Circulation. 1995;92:1720–5 at https://www.ahajournals.org/doi/10.1161/01.CIR.92.7.1720
- Servoss SJ, Januzzi JL, Muller JE. Triggers of acute coronary syndromes. Prog Cardiovas Dis. 2002;44:369–80 at https://www.ncbi.nlm.nih.gov/pubmed/12024335
- Williams JE, Nieto FJ, Sanford CP, Tyroler HA. Effects of an angry temperament on coronary heart disease risk: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2001;154:230–5 at https://www.ncbi.nlm.nih.gov/pubmed/11479187
- Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress [see comment] N Engl J Med. 2005;352:539–48 at https://www.nejm.org/doi/full/10.1056/nejmoa043046
- Jiang, W, Babyak M, Krantz DS. et al. Mental stress-induced myocardial ischemia and cardiac events. Journal of the American Medical Association,1996; 275:1651–1656 at https://www.ncbi.nlm.nih.gov/pubmed/8637138
- Modena, M, Corghi F, Fantini G et al.. Echocardiographic monitoring of mental stress test in ischemic heart disease. Clinical Cardiology 1989; 12, 21–24.at https://www.ncbi.nlm.nih.gov/pubmed/2912604
- Deanfield J, Shea M. Kensett M. et al. Silent myocardial infarction due to mental stress. Lancet 1984; 11, 1001–1004 at https://www.ncbi.nlm.nih.gov/pubmed/6149394
- Steptoe A and Brydon L Emotional triggering of cardiac events. Neuroscience and Biobehavioral Reviews, 2009; 33, 63–70 at https://www.ncbi.nlm.nih.gov/pubmed/18534677
- Fürst R, Zündorf I, Dingermann T. New Knowledge About Old Drugs: The Anti-Inflammatory Properties of Cardiac Glycosides. Planta Med 2017 Aug;83(12-13):977-984 at https://www.ncbi.nlm.nih.gov/pubmed/28297727
- Jagielska J, Salguero G, Schieffer B et al. Digitoxin elicits anti-inflammatory and vasoprotective properties in endothelial cells: Therapeutic implications for the treatment of atherosclerosis?, Atherosclerosis, 2009;206(2):390-6, 2009 at https://www.ncbi.nlm.nih.gov/pubmed/19446813
- Kolkhof P, Geerts A, Schäfer S et al. Cardiac glycosides potently inhibits C-reactive protein synthesis in human hepatocytes. Biochem Biophys Res Commun 2010, 26;394 (1): 233-9 at https://www.ncbi.nlm.nih.gov/pubmed/20206126
- Ihenetu K, Espinosa R, de Leon R et al. Digoxin and digoxin-like immunoreactive factors (DLIF) modulate the release of proinflammatory cytokines. Inflamm Res. 2008; 57(11): 519-23 at https://www.ncbi.nlm.nih.gov/pubmed/19109744
- Shah VO, Ferguson J, Hunsaker LA, Deck LM, Vander Jagt DL. Cardiac Glycosides Inhibit LPS-induced Activation of Proinflammatory Cytokines in Whole Blood through an NF-κB-dependent Mechanism. International Journal of Applied Research in Natural Products, 2011 Vol. 4 (1), pp. 11-19 at http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1009.6603&rep=rep1&type=pdf
- Yang Q, Huang W, Jozwik C, Lin Y, Glasman M et al. Cardiac glycosides inhibit TNF-alpha/NF-kappaB signaling by blocking recruitment of TNF receptor-associated death domain to the TNF receptor. Proc Natl Acad Sci USA. 5;102(27):9631-6, 2005 at http://www.pnas.org/cgi/content/full/102/27/9631
- Tani S, Takano R, Oishi S et al. Digoxin Attenuates Murine Experimental Colitis by Downregulating Th17-related Cytokines. Inflamm Bowel Dis. 2017, 23(5):728-738 at https://www.ncbi.nlm.nih.gov/pubmed/28426455
- Lee J, Baek S, Lee J et al, Digoxin ameliorates autoimmune arthritis via suppression of Th17 differentiation. International Immunopharmacol, 2015 V 26, Issue 1, 103–111 at https://www.ncbi.nlm.nih.gov/pubmed/25819229
- Gheorghiade M and Ferguson D. Digoxin, a neurohormonal modulator for heart failure? Circulation, 1991; V84:N5 at https://www.ahajournals.org/doi/10.1161/01.CIR.84.5.2181
- Fardin NM, Antonio EL, Montemor JA et al. Digitoxin improves cardiovascular autonomic control in rats with heart failure. Can J. Pharmacol, 2016; 94: 18 at https://www.ncbi.nlm.nih.gov/m/pubmed/27082032/
- Gutman Y and Boonyaviroj P. Mechanism of inhibition of catecholamine release from adrenal medulla by diphenylhydantoin and by low concentration of ouabain (10 (-10) M). Arch Pharmacol 1977,296(3);293-6:at https://link.springer.com/article/10.1007/BF00498696
- Kypson J Triner L, Nahas GG. The effects of cardiac glycosides and their interaction with catecholamines on glycolysis and glycogenolysis in skeletal muscle J Pharmacol Exp Ther, 1968;164(1): 22-30:1968 at http://jpet.aspetjournals.org/content/164/1/22.long
- Calderón-Montaño J, Burgos-Morón E, Lopez-Lazaro M. The Cardiac Glycosides Digitoxin, Digoxin and Ouabain Induce a Potent Inhibition of Glycolysis in Lung Cancer Cells. WebmedCentral CANCER 2013;,;4(7);WMC004323: 2013 at https://www.webmedcentral.com/wmcpdf/Article_WMC004323.pdf.
- Back M and Hansson GK. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation FASEB J. 2019; 33: 1536–1539 at https://www.fasebj.org/doi/10.1096/fj.201802445R
- Abuissa H, O’Keefe JH, Harris H et al. Autonomic Function, Omega-3, and Cardiovascular Risk. Chest Journal, 2005; Volume 127: Issue 4 at https://journal.chestnet.org/article/S0012-3692(15)34447-0/fulltext
- Ogilve GK, Fettman MJ, Mallinckrodt CH et al. Effect of fish oil, arginine, and doxorubicin chemotherapy on remission and survival time for dogs with lymphoma: A double-blind, randomized placebo-controlled study, Cancer, 2000; 88: 1016-28 at https://www.ncbi.nlm.nih.gov/pubmed/10760770
- Manzi L, Costantini L, Molinari L et al. Effect of Dietary w-3 Polyunsaturated Fatty Acid DHA on Glycolytic Enzymes and Warburg Phenotypes in Cancer, BioMed Research International. 2015; 7 pages Article ID 137097 at https://www.hindawi.com/journals/bmri/2015/137097/
- Menkin V. Studies on inflammation X. The cytolological picture of an inflammatory exudate in relation to its hydrogen’s ion concentration, Am J Pathol. 1934 Mar; 10(2): 193–210 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2062856/
- Wachman A, Bernstein DS. Diet and osteoporosis. 1968;1:958–9 at https://www.sciencedirect.com/science/article/pii/S0140673668909082
- Frassetto L, Banerjee T, Powe N et al. Acid Balance, Dietary Acid Load, and Bone Effects—A Controversial Subject. Nutrients 2018, 10, 517 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5946302/
- Monteiro CETB. Does Lactic Acidosis Cause Coronary Artery Calcification?
Positive Health Online 2020; Issue 259 at https://bit.ly/33920xA - Monteiro CETB. Acidic environment evoked by chronic stress: A novel mechanism to explain atherogenesis. Available from Infarct Combat Project, January 28, 2008 at http://www.infarctcombat.org/AcidityTheory.pdf
- Monteiro CETB. Acidity Theory of Atherosclerosis -- History, Pathophysiology, Therapeutics and Risk Factors – A Mini Review. Positive Health Online, Edition 226, November 2015 at http://goo.gl/AejGAV
- Monteiro CETB. Stress as Cause of Atherosclerosis – The Acidity Theory. pp.204-225 in PJ Rosch ed. “Fat and Cholesterol Don’t Cause Heart Attacks and Statins Are Not The Solution”, Published by Columbus Publishing Ltd, 2016 at https://www.amazon.com/Cholesterol-Cause-Attacks-Statins-Solution/dp/190779753X
- Altman N and Krzywinski M. Association, correlation and causation. Nature Methods, 2015; 12 : 899–900 at https://www.nature.com/articles/nmeth.3587
- Popper K.Conjectures and Refutations: The Growth of Scientific Knowledge 1963; pp. 33-39, London, Routledge at https://science.sciencemag.org/content/140/3567/643.1
- Rokitansky K: The Organs of Circulation: A Manual of Pathological Anatomy. Vol IV. 1855; Philadelphia, Blanchard & Lea at https://archive.org/details/manualofpatholog34rokirich
- Selye H. The Humoral Production of Cardiac Infarcts, British Medical Journal, March 15: 1958 at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2028103/pdf/brmedj03094-0021.pdf
Comments:
-
No Article Comments available