Positive Health Online
Your Country

Letters to the Editor Issue 275
listed in letters to the editor, originally published in issue 275 - January 2022
Using Nanofibers to Stop Brain Tumor Cells from Spreading
Researchers from Japan develop a platform based on nanofibers to trap brain cancer cells as a therapeutic strategy Brain tumour is difficult to contain and is often resistant to conventional treatment methods. Predicting tumour cell behaviour requires a better understanding of their invasion mechanism. Now, researchers from University of Fukui, Japan, have used high-density nanofibers that mimic the microenvironment of the brain to capture these tumour cells, opening doors to novel therapeutic solutions for aggressive brain cancer.
Our body heals its injuries by essentially replacing damaged cells with new cells. The new cells often migrate to the site of injury, a process known as “cell migration”. However, abnormal cell migration can also facilitate the transport and spread of cancer cells within the body. Glioblastoma multiforme (GBM) is one such example of a highly invasive brain tumour that spreads via migration of the tumour cells. The frequency at which such tumour cells spread and grow make conventional tumor removal methods ineffective. Furthermore, options such as radiotherapy and chemotherapy are harmful to healthy cells and cause adverse effects. In order to develop improved therapeutic strategies, a precise understanding of the invasion mechanism of GBM cells is necessary.
An alternative treatment strategy in consideration involves capturing the migrating tumour cells. It turns out that cell migration is dictated by the structure and the orientation of the “extracellular matrix” (ECM) – fibrous structures surrounding the cells. By engineering similar structures of desired geometries, it is, therefore, possible to exert control over the migration process.
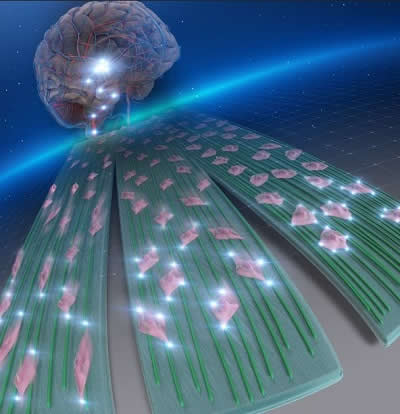
Detection of glioblastoma multiforme (GBM)
Glioblastoma multiforme (GBM) is an aggressive brain tumour that spreads along the white matter tracts of the brain. Now, researchers at University of Fukui, Japan, have managed to engineer nanofibers mimicking the brain that can stop them from spreading.
Photo credit: Cover for ACS Applied Bio Materials Vol. 4 No. 10, reused with permission from American Chemical Society
Now in a study published in ACS Applied Bio Materials, researchers from University of Fukui, Japan, have designed a platform based on nanofibers that resemble the ECM to examine their effect on GBM cells.[1]
“We fabricated a nanofibrous sheet in which the fiber density changes from end to end gradually using a technique called ‘electrospinning’ and carried out a culture experiment of brain tumor cells,” says Dr. Satoshi Fujita, who headed the study.
The researchers observed clear distinctions in cell movement in nanofibers of different densities. They found that the denser fibers promoted the formation of “focal adhesions” clusters in the cells that resulted in a slower cell migration.
Taking advantage of this negative correlation between cell movement and fiber density, the researchers were able to control and direct the migration of cells by designing a nanofibrous sheet with stepwise varying densities. By arranging the fibers in a high-to-low density configuration, they were able to restrict the movement of cells as most of them were captured in the high-density zones. On the other hand, a low-to-high density configuration had the opposite effect and encouraged migration.
In addition, they noticed that the gaps between the zones hindered cell migration, leading to cells being trapped in the high-density zones. This one-way migration was observed for the first time and the researchers named it “cell trapping” after fish and insect traps that cause their prey to travel along a single direction before trapping it.
“The study demonstrates the feasibility of capturing migrating cells using electrospun nanofibers that mimic the microenvironment of the brain,” comments Dr. Fujita.
With such remarkable findings, the team is excited about the future prospects of their nanofiber-based platform.
“It is available for the design of scaffolding materials, which are the basis of regenerative medicine, in combination with various fiber processing technologies and material surface treatment technologies. This could lead to the development of practical applications of regenerative medicines,” speculates Dr. Fujita, “In addition, it can be used as a processing technology for culture carriers for efficient production of biological drugs including proteins, antibodies, and vaccines.”
We certainly hope his visions are realized soon!
Reference
- Wan-Ying Huang, Shin-ichiro Suye, and Satoshi Fujita. Cell Trapping via Migratory Inhibition within Density-Tuned Electrospun Nanofibers. ACS Applied Bio Materials 4 (10): 7456-7466. September 3, 2021. DOI: https://doi.org/10.1021/acsabm.1c00700
About University of Fukui, Japan
The University of Fukui is a preeminent research institution with robust undergraduate and graduate schools focusing on education, medical and science, engineering, and global and community studies. The university conducts cutting-edge research, including fiber and textile engineering, and strives to nurture human resources capable of contributing to society on the local, national, and global level.
Website: https://www.u-fukui.ac.jp/eng/
About Dr. Satoshi Fujita from University of Fukui, Japan
Dr Satoshi Fujita is an Associate Professor at the Department of Frontier Fiber Technology and Science at University of Fukui, Japan. His research interests comprise nanofibers, biopolymers, biomaterials and their applications for tissue engineering, medical devices and drug delivery systems. He has published over 40 papers and has 7 patents under his name.
Funding information
This study was funded by Terumo Life Science Foundation and the Grant-in-Aid for Scientific Research (C) (19K09502, 21K09172) from the Japan Society for the Promotion of Science.
Media Contact:
Mika Hayashi sskoho-k@ad.u-fukui.ac.jp
Diabetes Mellitus as Risk Factor for Glaucoma The Underlying Cause
by Carlos ETB Monteiro
“The high prevalence of autonomic dysfunction in patients with shallow anterior chambers and glaucoma may explain this association. Because of this, acute glaucoma should be regarded as a symptom of diabetes.”[1] by Mapstone R and Clark VC, 1985[1]
Introduction
John R. Armstrong and colleagues, in 1960,[2] following patients of the diabetic clinic of Jefferson Davis Hospital, have noticed a high incidence of concurrent ocular disease. They observed that such conditions as cataract, diabetic retinopathy, rubeosis iridis, venous thrombosis and hemorrhage occurred much more frequently in diabetics than in nondiabetics. However, the diagnoses of glaucoma also appeared more often among the diabetics of their clinic than might be expected in non-diabetics. When standard texts on ophthalmology and on diabetes were examined, there was no mention of an increased incidence of glaucoma in diabetes; therefore, a more extensive survey of the available literature was done. In order that a proper comparison might be made between the diabetic and nondiabetic populations, the reported incidence of glaucoma in the nondiabetic population was ascertained. The disparity of statistics by the authors just mentioned and the aforementioned clinical impression stimulated interest in further investigation and a study, designed to illuminate any relationship between glaucoma and diabetes, was undertaken. Those patients with suspicious tensions were referred to the glaucoma clinic of Jefferson Davis Hospital for further studies. Of the 393 diabetics, 4.1 percent were found to have primary, non-congestive glaucoma, and 1.8 percent were found to have secondary glaucoma. In a majority of cases, diabetes was discovered before glaucoma.
After the discovery from Armstrong and colleagues[2} other studies also confirmed the association between Diabetes and Glaucoma. For example:
- A study from 1994[3] concluded: “The presence of open-angle glaucoma is increased in people with older-onset diabetes”;
- A study from 1997[4] concluded: “The significant and consistent association between diabetes and glaucoma found in our study, which appeared independent of the effect of diabetes on intraocular pressure, suggests that there is a real association between these two diseases”;
- A study from 2014 [5] concluded: Individuals with Diabetes Mellitus have an increased risk of developing primary open-angle glaucoma;
- A Meta- analysis, published in 2017,[6] has involved seven prospective studies published between 2000 till 2014. The authors told; “The results of this review revealed that the incidence of glaucoma markedly increased by 36% (RR=1.36, 95%CI:1.25-1.50) in patients with diabetes compared with individuals with no diabetes.” In conclusion they said: “Diabetes is associated with significantly increased risk of glaucoma”
The Underlying Cause
According to our concept, born in 2006[7] the autonomic dysfunction may accelerate glycolysis, what results in increased lactate in many diseases. This led us to many studies including about diabetes mellitus,[8] and hypertension.[9] The history about the development of our present concept was recently published.[10]
Autonomic Dysfunction and Glaucoma
However, many studies have shown that autonomic dysfunction is a factor in the development of Glaucoma, not depending exclusively on Diabetes, hypertension or others associated diseases. Follows some of these studies:
- A study from 1981[11] told that one of the functions of the autonomic nervous system is the regulation of the intraocular pressure. This is carried out partly by the sympathetic and partly by the parasympathetic system. An understanding of this regulatory process therefore requires an understanding of the anatomy, physiology, and pharmacology of the sympathetic and parasympathetic innervation of the eye;
- A study from 2019[12] found that glaucoma patients with autonomic dysfunction or optic disc hemorrhage have larger baseline pupils in darkness and different pupil constriction responses. However, the authors told they should keep in mind that assessment of pupil responsiveness would not be enough to conclude for the presence of autonomic neuropathy;
- A study from 2019[13] aimed to investigate changes in Schlemm’s canal, intraocular pressure (IOP) and ocular blood circulation following the activation of the sympathetic nervous system. Twenty healthy volunteers were enrolled in this study. The cold pressor test (CPT) was adopted. The expansion of Schlemm’s canal was observed after the CPT might be caused by sympathetic nerve stimulation, subsequently leading to decreased IOP;
- A study from 2020[14] says that in meta-analyses, it has been reported that myopia is a risk factor for glaucoma and there is increasing evidence that autonomic dysfunction causing vascular dysregulation or perfusion dysfunction is considered an important factor in the progression of glaucoma. There have been experimental studies to find out the association between autonomic nervous system and ocular growth, but no clinical study yet has evaluated the relationship between them. Therefore, they enrolled 208 open angle glaucoma patients and measured heart-rate-variability (HRV). The authors suggested the possibility of association between myopic deformation and autonomic dysfunction;
- Another study from 2020 [15) found that Low HRV, high SBP, high PP, and hypertension were associated with glaucoma. Longitudinal studies may elucidate if autonomic dysregulation and high BP also predict glaucoma incidence.
Glycolysis and Glaucoma
- A study from 2019 [16] found elevated expression levels of MCT1, GLS2 and MTFDH2, indicating enhanced glycolysis and glutaminolysis in glaucomatous LC cells. They said this is novel evidence that glaucomatous LC cells utilize alternative metabolic pathways. Blocking these pathological pathways would be a potential therapeutic in glaucoma;
- A study published in 2020 [17] demonstrated evidence of metabolic reprogramming (The Warburg effect) in glaucoma LC cells. Expression of markers of glycolysis, glutamine, and one carbon metabolism are elevated in glaucoma cells at both the mRNA and protein levels. A better understanding of bioenergetics in glaucoma may help in the development of new therapeutics.
Digitalis and Glaucoma
Digitalis and other cardiac glycosides, at daily low concentration doses, are potential drugs to fight Glaucoma.[18, 19, 20). This, in our concept, by restoring the balance of the autonomic nervous system and reducing the production of lactic acid in the body.[8,9]
References
- Mapstone R and Clark VC. Prevalence of diabetes in glaucoma. British Medical Journal V 291 13 July 1985 at https://www.bmj.com/content/291/6488/93
- Armstrong JR, Daily RK, Dobson HL, Obson HL and Girard LJ. The incidence of Glaucoma in Diabetes Mellitus. A comparison with the incidence of glaucoma in the general population. Am J Ophthalmol. 1960 ;50:55-63 at https://www.ajo.com/article/0002-9394(60)90840-0/pdf
- Klein BEK, klein R and Jensen SC. Open angle Glaucoma and Older onset Diabetes. The Beaver Dam Eye Study. Ophthalmology 1994; 10 1: 1173-1177 at https://www.aaojournal.org/article/S0161-6420(94)31191-2/pdf
- Mitchell P, Smith W et al. Open--angle Glaucoma and Diabetes: The Blue Mountains Eye Study, Australia. Ophthalmology 1997; 104:712-718 at https://www.sciencedirect.com/science/article/abs/pii/S0161642097302474
- Minwen Zhou, Wei Wang, Wenbin Huang and Xiulan Zhang. Diabetes Mellitus as a Risk Factor for Open-Angle Glaucoma: A Systematic Review and Meta-Analysis. PLOS ONE Volume 9 , Issue 8; e102972 2014 at https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0102972
- Zhao YX, Chen XW. Diabetes and risk of glaucoma: systematic review and a Meta-analysis of prospective cohort studies. Int J Ophthalmol 2017;10(9):1430-1435 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5596230/
- Monteiro CETB. Acidic environment evoked by chronic stress: A novel mechanism to explain atherogenesis. Available from Infarct Combat Project, January 28; 2008 at http://www.infarctcombat.org/AcidityTheory.pdf
- Monteiro CETB. The Causal Role of Autonomic Dysfunction and Lactic Acidosis in the Development of Diabetes Mellitus, Positive Health Online Issue 275 - January 2022 at http://www.positivehealth.com/article/diabetes/the-causal-role-of-autonomic-dysfunction-and-lactic-acidosis-in-the-development-of-diabetes-mellitus-1
- Monteiro CETB. The Causal Role of Autonomic Dysfunction and Lactic Acidosis in the Development of Hypertension. Positive Health Online issue 271 - June 2021 at http://www.positivehealth.com/article/heart/the-causal-role-of-autonomic-dysfunction-and-lactic-acidosis-in-the-development-of-hypertension
- Monteiro CETB. “Looking for the Cause and Cure of Diseases -- Possible Mechanisms Underlying the Relationship of Stress to Disease” Contentment Magazine: Spring 2021 at https://www.stress.org/looking-for-the-cause-and-cure-of-diseases-possible-mechanisms-underlying-the-relationship-of-stress-to-disease
- Hoyng P.F.J. (1981) The Autonomic Nervous System and the Intraocular Pressure. In: Pharmacological Denervation and Glaucoma. Monographs in Ophthalmology 2, vol 2. Springer, Dordrecht at https://link.springer.com/chapter/10.1007/978-94-009-8674-9_2
- Hae-Young Lopilly Park,, Suk Hoon Jung, Sung-Hwan Park and Chan Kee Park. Detecting autonomic dysfunction in patients with glaucoma using dynamic pupillometry. Medicine (2019) 98:11 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6426567/
- Wei Chen, Zhiqi Chen, Yan Xiang, Chaohua Deng, Hong Zhang and Junming Wang. Simultaneous influence of sympathetic autonomic stress on Schlemm’s canal, intraocular pressure and ocular circulation. Scientific Reports | (2019) 9:20060 at https://www.nature.com/articles/s41598-019-56562-0.pdf
- DaYoung Shin, Soo Ji Jeon, HaeYoung Lopilly Park & Chan Kee Park. Posterior scleral deformation and autonomic dysfunction in normal tension glaucoma. Scientific Reports (2020) 10:8203 at https://www.nature.com/articles/s41598-020-65037-6.pdf
- Nigus G. Asefa; Anna Neustaeter; Nomdo M. Jansonius; Harold Snieder. Autonomic Dysfunction and Blood Pressure in Glaucoma Patients: The Lifelines Cohort Study. Investigative Ophthalmology & Visual Science September 2020, Vol.61, 25 at https://iovs.arvojournals.org/article.aspx?articleid=2770802
- Mustapha Irnaten, Diarmaid Hickey, Deirdre Brennan, et al. Evidence of Increased Glycolysis and Glutaminolysis in Glaucoma Lamina Cribrosa Cells. Investigative Ophthalmology & Visual Science. ARVO Annual Meeting Abstract July 2019, Vol.60, 5650 at https://iovs.arvojournals.org/article.aspx?articleid=2744867
- Khalid Kamel, Colm J. O'Brien, Alexander V. Zhdanov et al. Reduced Oxidative Phosphorylation and Increased Glycolysis in Human Glaucoma Lamina Cribrosa Cells. Investigative Ophthalmology & Visual Science November 2020, Vol.61, 4 at https://iovs.arvojournals.org/article.aspx?articleid=2771917
- Simon Kenneth A. and Bonting Sjoerd L. Possible Usefulness of Cardiac Glycosides in Treatment of Glaucoma. Archives of Ophthalmology, Volume 68 (2) – Aug 1, 1962 at https://jamanetwork.com/journals/jamaophthalmology/article-abstract/626951
- W. Hardt, R. Johnen and M. Fahle. The Influence of Systemic Digitalis Application on Intraocular Pressure. Graefe's Arch Clin Exp Ophthalmol (1982) 219:76-79 at https://pubmed.ncbi.nlm.nih.gov/7141233/
- Adriana Katza, Daniel M. Tala, Dan Hellerb et al. Digoxin derivatives with selectivity for the α2β3 isoform of Na,K-ATPase potently reduce intraocular pressure. PNAS, November 3, 2015; vol. 112: no. 44 at https://www.pnas.org/content/112/44/13723
Further Information
Please contact Carlos ETB Monteiro via cmonteiro_icp@yahoo.com
Impulse Dynamics Announces First Optimizer Implant In China
Impulse Dynamics, a global medical device company dedicated to improving the lives of people with heart failure, today announced the first implantation of a patient in China with its innovative Optimizer system delivering cardiac contractility modulation – CCM therapy. Cardiologist Dr Hua Wei from the renowned Fuwai Hospital of the Chinese Academy of Medical Sciences performed the successful implant procedure
Dr Wei shared, “We are currently relying on medication only for the treatment of heart failure. If medication doesn’t work, then we don’t have a better solution. We now have a new option – CCM therapy – for these patients with a narrow QRS heart failure [QRS duration represents the time for ventricular depolarization]. For the vast majority of patients with heart failure, I think the implant performed today has a significant meaning for the future.”
The Optimizer, harnessing advanced engineering, delivers CCM therapy – the company’s proprietary technology – to the heart. CCM therapy has been pioneered by Impulse Dynamics to significantly improve heart contraction, allowing more oxygen-rich blood to be pushed throughout the body.[1] The Optimizer delivers precisely timed electrical pulses to the heart during the absolute refractory period of the beating cycle, immediately after the heart contracts. This breakthrough device improves the quality of life for heart failure patients no longer able to benefit from medication meant to manage symptoms and slow the progression of their condition.[1]
“Following years of collaboration between accomplished Chinese clinicians and our local team to obtain market authorization, we are happy to be offering the breakthrough technology of CCM therapy to Chinese patients and their families to restore their quality of life and help them regain hope” said Simos Kedikoglou, CEO of Impulse Dynamics.
Reference
- European Journal of Heart Failure. https://doi.org/10.1002/ejhf.2202.
About Impulse Dynamics
Impulse Dynamics, based in Marlton, N.J., is dedicated to helping healthcare providers enhance the lives of people with heart failure by transforming how the condition is treated. The company has pioneered CCM® therapy, which is delivered by the company’s Optimizer® system, a breakthrough, FDA-approved treatment verified to improve the quality of life for heart failure patients. CCM therapy is a safe and effective minimally invasive treatment option for many heart failure patients who otherwise have few treatment alternatives available to them. To learn more, visit www.ImpulseDynamics.com.
Contact and Further Information
Rex Richmond, Director Media Relations, Impulse Dynamics rrichmond@impulsedynamics.com
Tel: 856-642-9933 rrichmond@impulsedynamics.com www.ImpulseDynamics.com
Gerry Haines, CFO (Investor Relations) ghaines@impulsedynamics.com
Impulse Dynamics
Tel: 856-642-9933 www.ImpulseDynamics.com
Ian Segal, Public and Media Relations Manager
Ian Segal isegal@impulsedynamics.com
Impulse Dynamics
Tel: 856-642-9933 www.ImpulseDynamics.com
Review of 12 Intervention Trials Concludes Vitamin C Works for COVID. Why aren’t we being told to take it?
A review of 12 vitamin C studies, including five randomised controlled trials, is published in the journal Life today (Monday 1st Nov) showing that this simple, cheap vitamin can, and is, saving lives when given in the right dose.
Everyone knows that vitamin C is important for immunity – sales of both oranges and vitamin C tablets have risen sharply during the Covid pandemic.
But can vitamin C actually treat or prevent Covid infection from becoming serious? A review of twelve studies, including five ‘gold standard’ randomized controlled trials, shows that this simple vitamin can, and is, saving lives when given in the right dose.
The scientific evidence is clear; vitamin C can potentially help prevent Covid and, if taken once infected, can also reduce symptoms and duration of illness. So why aren’t we being told to supplement with vitamin C?
The review of the 12 studies, including the five randomised controlled trials, is published in the journal Life, [www.vitaminC4covid.com/12trialreview], and was carried out and funded by VitaminC4Covid.
VitaminC4Covid is a team of vitamin C experts including Dr Marcela Vizcaychipi from the Faculty of Medicine at London’s Imperial College, and Associate Professor Anitra Carr who heads the Nutrition in Medicine group at the University of Otago where they have been monitoring all Covid-related studies on vitamin C.
The studies show that Covid patients have depleted vitamin C levels, often to the level found in scurvy, and need substantial doses to recover and survive.
Dr Vizcaychipi, who heads research in intensive care medicine at the Chelsea & Westminster Hospital has been giving Covid and non-Covid patients in their Intensive Care Units up to 6 grams of vitamin C intravenously. The dosage is dependent on the severity of disease and the amount needed to correct deficiency, as indicated by vitamin C urine sticks (when available).
“Vitamin C is certainly one of multiple factors that contributes to better outcomes and speed of recovery. It should be standard practice. We have not had any safety issues at all.” says Dr Vizcaychipi, who heads research in intensive care medicine at the Chelsea & Westminster Hospital.
What the review of 12 clinical trials shows is that “intravenous vitamin C may improve oxygenation parameters, reduce inflammatory markers, decrease days in hospital and reduce mortality, particularly in the more severely ill patients.”
What is remarkable about vitamin C is that it is both an antioxidant (meaning it protects oxygen supply), an anti-viral, and is also anti-inflammatory – so an impressive three-in-one defender. Not one adverse event has been reported in any published vitamin C clinical trials in COVID-19 patients.
Also, the review shows that high doses of vitamin C tablets upon infection may also keep people out of hospital through increasing their rate of recovery.
According to Carr
“Oral doses of 8 grams per day have been shown to increase the rate of recovery from symptomatic infection by 70%. For more critically ill patients trials using doses of 6-24g a day intravenously have shown positive benefits in terms of increased survival, and reduced hospital stay, improved oxygenation or reduced inflammation."
Twenty oranges provide one gram of vitamin C, so these kinds of levels require supplementation. The review includes several studies showing that “patients with severe respiratory infections have depleted vitamin C status, with the prevalence of deficiency increasing with the severity of the condition”.
In one study vitamin C levels predicted who would or wouldn’t survive. Plasma concentrations of vitamin C were reported to be very low in 70-80% of Covid patients. What is clear is that several grams, not just a glass of orange juice, are needed to correct vitamin C deficiency. Dr Vizcaychipi has used inexpensive vitamin C urine sticks to check if enough is being given to reload vital vitamin C stores.
The VitaminC4covid team, founded by nutrition expert Patrick Holford, have been calling on government advisors to carry out a proper review of the evidence for vitamin C, in order to inform the public and medical profession, for over a year.
NICE (the National Institute for Health and Care Evidence) set up the RAPID C-19 oversight group exactly for the purpose of finding potential treatments as fast as possible. Yet, a NICE spokesperson confirmed that “neither NICE nor RAPID C-19 have undertaken a review of vitamin C for COVID-19” since September 2020, when these trials were initiated and noted by RAPID C-19.
To make matters worse a Freedom of Information request revealed that RAPID C-19 told Public Health England, which oversees the nation’s health education for disease prevention, specifically not to review vitamin C [or D] to avoid “duplication of effort”.
So, to date, no government agency has reviewed the evidence from a growing number of well-designed clinical trials. Despite this MPs were recently told by Jo Churchill, the Parliamentary Under Secretary of State for Primary Care and Health Promotion that “we do not believe that there is sufficient evidence at this stage to conclude that vitamin C is a safe and effective treatment for COVID-19”.
How does she form such a belief when no review has been carried out? A UK trial set up in June 2020, called REMAP-CAP, has not even started due, they say, to “lack of vitamin C”. There is no shortage.
“There seems to be a double standard” says Holford. “The promise of evidence-based medicine to those advocating non-drug treatments such as vitamins was effectively – come up with the evidence and we’ll treat it like any other medicine. The evidence is now undeniable, so why aren’t people being told to take high dose vitamin C upon infection and all hospitals checking vitamin C status with urine sticks as a routine measure and acting accordingly? Vitamin C is safe, inexpensive, available and now proven to work.”
Further Information
See www.vitaminC4covid.com/recommendations for guidance on what to take for prevention and if you become infected.
To access the full review, see: www.vitaminC4covid.com/12trialreview
For more information on the current NHS misinformation around vitamin C see: https://healthinsightuk.org/2021/10/29/vitamin-c-saga-how-a-small-expert-group-fought-back-against-nhs-misinformation/
Media Enquiries
For media enquiries please contact Sophie at Panpathic Communications on Tel: 07815 860 082; 01323 440 998; Sophie@panpathic.com
Summary of Recent Research with Three Nutrients in the War Against Cancer
by Michael Passwater
Click here to see a web copy of this news release
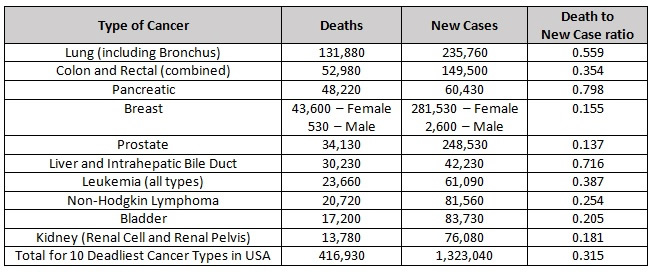
We have much to learn about preventing and treating cancer. Each disease in the cohort of cancers is potentially a different disease related to the specific genetic mutations that drive it. In the interest of promoting further efforts to improve our understanding of these complex diseases, here is an update on research involving three essential nutrients: vitamin C, selenium (selenite and methyl selenocysteine), and vitamin E (delta tocotrienol). Although much progress has been made in the fight against some types of cancer, many types still remain literally a death sentence. Each year, more than 1.3 million people are diagnosed with one of the ten most deadly types of cancer in the United States and over 400,000 people die from these dreaded diseases.
2021 Estimated Cancer Deaths and New Cases in the United States [1]
Ascorbic Acid (Vitamin C)
By definition, a vitamin is essential for life, and vitamin C (ascorbic acid) is no exception. The health benefits of vitamin C are so extensive that it would be an important tool in the battle against cancer even without its powerful anti-tumor effect. Vitamin C is important for many aspects of immune and endothelial health; synthesis of catecholamines, carnitine, neuropeptides, neurotransmitters, collagen, and elastin; breakdown of L-tyrosine and hypoxic inducible factor 1-alpha (HIF-1a); epigenomic regulation; somatic stem cell reprogramming; and redox regulation including scavenging damaging free radicals, breaking chains of lipid peroxidation, and recycling other antioxidants. [2-8]
In 1976, Cameron and Pauling reported longer survival in terminal cancer patients given vitamin C. [9] Was this from improvement to the patients' constitution, was there an anti-tumor effect, or both? We now know that high doses of vitamin C can have a prooxidant effect, especially in combination with iron, which can selectively kill tumor cells containing less catalase than healthy human cells. [10, 11] The catalase enzyme is necessary for nearly all cells to remove hydrogen peroxide that can cause free radicals and damage the cell's biomolecules, including proteins and DNA. And vitamin C can also disrupt the "Warburg Effect" in tumor cells. [12,13,14] The Warburg Effect is the tendency of tumor cells to switch from using mitochondrial oxidative phosphorylation to aerobic glycolysis for the production of cellular energy (ATP). While greatly reducing the efficiency of energy production (2 ATP molecules generated per glucose molecule vs. 36 ATP molecules generated per glucose molecule), aerobic glycolysis enhances the ability of the cell to proliferate. Efficiency is only important when resources are scarce. In a glucose rich environment, 2 ATP molecules per glucose will work just fine, and allows more of the glucose, along with the amino acid glutamine, to provide the structures necessary to make additional tumor cells. This primitive, yet streamlined, tumor metabolism allows abundantly available glucose and glutamine to supply the biochemical needs of cell growth and division. Many tumor cells over-express glucose transporters, particularly GLUT1 to increase intake of glucose. Ascorbic acid, and especially oxidized ascorbic acid (dehydroascorbic acid) is similar in molecular shape to glucose, and can enter cells through these membrane transport channels. Not only does this disrupt the supply of glucose to the tumor cell, it also allows increased vitamin C concentrations within the cell where it can cause epigenetic effects including increasing TET enzyme activity, re-expression of tumor suppressor genes, or cause cell death through metabolic disruption. Depletion of intracellular glutathione leads to inactivation of glyceraldehyde 3-phosphate dehydrogenase and inhibition of glycolysis. In the setting of high vitamin C concentrations, the reliance of some tumor cells on aerobic glycolysis becomes their "Achilles' heel". [15]
In recent years, cell culture studies and human clinical trials have confirmed that tumor cells with KRAS and BRAF mutations can be selectively killed by appropriate doses of vitamin C. [16] KRAS and BRAF mutations are common in solid tumors, especially pancreatic, colon, and lung cancers. Synergistic effects have been seen by combining vitamin C with some chemotherapy drugs, radiation treatment, or in combination with a fasting-mimicking diet. [17-23] Mark Levine, Channing Paller, Tami Tamashiro, Thomas Luechtefeld and Amy Gravell recently reviewed 53 cancer clinical trials involving IV and/or oral vitamin C. [15] These trials give a clear signal of safety when patients with glucose 6 phosphate dehydrogenase (G6PD) deficiency are excluded from high dose IVC administration. Most studies were small, and involved a wide variety of late stage cancers. Nonetheless, encouraging signals were seen, including a few patients surviving years later eventually dying of causes other than cancer. Studies involving pancreatic ductal adenocarcinoma (PDAC) show a median survival following diagnosis of 3.5 months untreated, 6.7 months when treated with gemcitabine, 8.5 - 13 months when treated with gemcitabine and nab-paclitaxel, and 15.1 months when IVC with or without gemcitabine was used. [17,24] It is likely that with refinement of dosing and route of administration, earlier intervention, and improved knowledge of which tumors are most susceptible to high dose vitamin C therapy, more consistent positive results will be seen.
Three promising active clinical trials:
A Phase II Trial of Pharmacological Ascorbate, Gemcitabine, and Nab-Paclitaxel for Metastatic Pancreatic Cancer (PACMAN 2.1; University of Iowa Holden Comprehensive Cancer Center) [25]
A Phase II Study of High Dose Vitamin C Intravenous Infusion in Patients with Resectable or Metastatic Solid Tumor Malignancies (Weill Cornell Medicine, NYC) [26]
To download an informative description of "IV Vitamin C for Cancer Care", please click https://riordanclinic.org/wp-content/uploads/2017/09/IVChandout.pdf.
(Riordan Clinic, Kansas, >250,000 IVC treatments provided) [27]
Selenium
Selenium is a micronutrient essential for human health. There are 25 human selenoproteins known to be involved in numerous functions throughout the body, including brain, blood vessel, heart, and immune system health. These proteins perform diverse functions including antioxidant and redox recycling, gene "proof-reading", vitamin D metabolism, and hemostasis. Like vitamin C, even if there were no direct tumor effect of selenium, it would be an important tool in the battle for wellness and against cancer. [28-31]
HLA-E is a "camouflage protein" expressed by some tumors to hide from the immune system. The protein fools the immune system into thinking the tumor cell is a normal cell. A decade ago, selenite was shown to reduce - nearly eliminate - HLA-E expression on tumor cells. The tumor cells then became susceptible to destruction by Natural Killer cells (immune cells which clear unhealthy cells from the body). [32] A recent study of 243 PDAC patients showed that higher HLA-E and HLA-G (a similar protein) expression was associated with shorter survival. [33] In addition to optimizing tissue health throughout the body, the reduction of camouflage membrane proteins HLA-E and HLA-G by selenite may be a useful tool in the battle against cancer. Of note, selenomethionine (SeMet) had no effect on tumor cell HLA-E expression. Selenomethionine was the form of selenium used in the SELECT trial which did not show a benefit of selenium against prostate cancer prevention. In addition to the unfortunate choice of selenium supplement, the trial had other flaws, including a lack of difference in selenium levels between the control and test groups. Methyl selenocysteine (MSC) was the form of selenium used in the Nutritional Prevention of Cancer (NPC) trial which showed benefit. MSC is also the dominant form of selenium in broccoli, cabbage, onions and garlic. In 2014, MSC was shown to protect against the toxicity of four chemotherapy agents (cyclophosphamide, cisplatin, oxalplatin, and irinotecan), and enhance antitumor activity. [34]
More recently, a phase I study at Stanford using selenite and radiation therapy in humans validated the further study of selenite against cancer. [35] Additionally, in vitro and in vivo (mice) studies at the University of Grenada showed a strong antitumor effect of selenite against pancreatic cancer, alone and in combination with gemcitabine. [36]
Delta Tocotrienol
Vitamin E is an essential nutrient for humans. Vitamin E is a lipid soluble antioxidant. There are 8 different molecules in the vitamin E family pertinent to humans. It comprises 4 tocopherols (alpha, beta, gamma, delta), and 4 tocotreinols (alpha, beta, gamma, delta). Tocotreinols are smaller than tocopherols and are unsaturated. Tocotrienols have a half-life of approximately 4 hours while tocopherols have a half-life of 20 hours. Delta tocotrienol (VEDT = Vitamin E Delta Tocotrienol) is the smallest member of the vitamin E family, as it has the shortest "tail". Its small size allows delta tocotrienol greater mobility within lipid layers of cell membranes.
Studies have shown that tocotreinols inhibit Nuclear factor kappa B (NF-kB) activity and human pancreatic cancer cell survival, with VEDT having the strongest effect. [37] NF-kB is involved in immune and inflammatory responses, and in the regulation of cellular gene expression, proliferation, differentiation, and survival. VEDT has also been shown to enhance gemcitabine activity in pancreatic cancer cells. [38-40] Furthermore, VEDT has been shown to inhibit PDAC stem-like cells. VEDT significantly inhibited growth and metastases of these cells, including inhibiting the growth and metastasis of gemcitabine resistant PDAC stem-cell-like cells. [41] A phase I human clinical trial at Moffitt Cancer Center in Tampa, Florida showed 200 to 1600 mg VEDT taken orally daily for 2 weeks was well tolerated and significantly induced apoptosis (cell death) in pancreatic ductal cancer cells. [42] An anti-tumor effect, as well as a reduction in side effects from chemotherapy, has also been shown in the setting of other solid tumors. [43-56]
Note that to prevent the larger tocopherols from blocking absorption of the smaller tocotreinols, it is best to avoid taking tocopherols at the same time as tocotrienols. [57-63] Twice daily dosing of tocotrienols have been shown to produce a steady state after three days.
Summary
The redox synergy of vitamin C, vitamin E, and selenium-containing glutathione peroxidases has been explored since the 1960s and 1970s. Studies focusing on single nutrient interventions, with and without chemotherapy and radiation, have suggested benefit in humans, with increasingly specific mechanistic and treatment details discovered each decade. Along the way, a reassuring safety profile of nutrient interventions with and without traditional chemotherapy and radiation interventions has been established. Further studies to better define the most effective match of treatment for each tumor type, along with the best route, dose, and combination of treatments are important next steps to improve the reliability and effectiveness of preventing and treating the many types of cancer. Combining nutrients known to be synergistic together, while limiting glucose and glutamine central to the metabolism of many tumor cells, may optimize the effectiveness of treatment strategies.
With nutrient therapy - orthomolecular medicine - one does not need to choose between attacking the tumor and strengthening the host. The treatments often impact both simultaneously.
Foundational Support
- NAD deficiency is associated with greater risk for mutagenesis with cancer and this is likely best avoided using daily niacin, e.g. start with 3x25-100mg/d to adapt to the flush, and then slowly work up to 3x500-1,000mg/d. [64] You can start by purchasing 100 mg tablets and break them into halves or quarters at first.
- For cancer patients, chemotherapy commonly causes NAD deficiency, which is best rescued with niacinamide; e.g. 3x500mg/d. [64]
- Dietary relevance, glutamine restriction with niacinamide; glucose restriction and ketogenic diet is recommended. [64]
- Vitamin D3 to maintain plasma vitamin D > 40 ng/ml. Test a dose of 125 mcg/d (5000 IU) D3 with 100 mcg/d K2 for 4 months before getting a blood test for vitamin D.
- 100 - 200 mcg/d selenium as methyl-selenocysteine or selenium yeast
- 300 mg/d alpha lipoic acid
- 1-2 g vitamin C with each meal
- Exercise
- Cultivate a cheerful heart [65]
Advanced Support
Vitamin C in divided doses throughout the day to bowl tolerance; consider mixtures of lipid- and water-soluble forms
- IVC if G6PD levels are adequate and IVC is available from a licensed medical provider [66]
- Sodium selenite if available from a licensed medical provider
- Delta tocotrienol 300 - 1600 mg/d [42]
(Michael Passwater is certified by the American Society for Clinical Pathology as a Medical Technologist, a specialist in Immunohematology, and a diplomate in Laboratory Management. He has worked in clinical laboratories for 28 years, and has his degree in Medical Technology from the University of Delaware.)
References & Further Reading
- National Cancer Institute. Common Cancer Types. https://www.cancer.gov/types/common-cancers
- Oudemans-van Straaten HM, Spoelstra-de Man AME, de Waard MC. (2014) Vitamin C revisited. Critical Care 18:460-473. https://pubmed.ncbi.nlm.nih.gov/25185110
- Manning J, Mitchell B, Appaduras DA, May JM, et al. (2013) Vitamin C Promotes Maturation of T-Cells. Antioxid Redox Signal. 19:2054-2067. https://pubmed.ncbi.nlm.nih.gov/23249337
- Ladumer A, Schmitt CA, Schachner D, et al. (2012) Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med. 2012 May 15; 52:2082-2090. https://pubmed.ncbi.nlm.nih.gov/22542797
- May JM, Qu ZC. (2010) Ascorbic Acid Prevents Increased Endothelial Permeability Caused by Oxidized Low Density Lipoprotein. Free Radical Res. 44:1359-1368. https://pubmed.ncbi.nlm.nih.gov/20815791
- Deicher R, Ziai F, Begknayer C, et al. (2005) Low Total Vitamin C Plasma Level Is a Risk Factor for Cardiovascular Morbidity and Mortality in Hemodialysis Patients. J Am Soc Nephrol. 16:1811-1818. https://pubmed.ncbi.nlm.nih.gov/15814831
- Heller R, Munscher-Paulig F, Grabner R, Till V. (1999) L-Ascorbic Acid Potentiates Nitric Oxide Synthesis in Endothelial Cells. J Biol Chem, 274:8254-8260. https://pubmed.ncbi.nlm.nih.gov/10075731
- Leibovitz B, Siegel BV. (1978) Ascorbic acid, neutrophil function, and the immune response. Int J Vitam Nutr Res. 48:159-164. https://pubmed.ncbi.nlm.nih.gov/357320
- Cameron E, Pauling L. (1976) Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 73: 3685-3689. https://pubmed.ncbi.nlm.nih.gov/1068480
- Schoenfeld JD, Sibenaller ZA Mapuskar KA, et al (2017) O2- and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell 31: 487-500. https://pubmed.ncbi.nlm.nih.gov/28366679
- O'Leary BR, Alexander MS, Du J, et al (2020) Pharmacological ascorbate inhibits pancreatic cancer metastases via a peroxide-mediated mechanism. Sci Rep 10:17649 https://pubmed.ncbi.nlm.nih.gov/33077776.
- Aguilera O, Muñoz-Sagastibelza M, Torrejón E, et al. (2016) Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget, 7:47954-47965. https://pubmed.ncbi.nlm.nih.gov/27323830
- Suzuki T, Kishikawa T, Sato T, et al. (2021) Mutant KRAS drives metabolic reprogramming and autophagic flux in premalignant pancreatic cells. Cancer Gene Ther. Online ahead of print. https://pubmed.ncbi.nlm.nih.gov/33833413
- Gonzalez MJ, Seyfried T; Nicolson GL et al. (2018) Mitochondrial Correction: A New Therapeutic Paradigm for Cancer and Degenerative Diseases. J Orthomolecular Med 33: 4. https://riordanclinic.org/journal-article-archive/mitochondrial-correction-a-new-therapeutic-paradigm-for-cancer-and-degenerative-diseases
- Chen Q, Vissers MCM (2020) Cancer and Vitamin C. CRC Press. ISBN-13: 978-0367858049
- Yun J, Mullarky E, Lu C, et al. (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350:1391-1396. https://pubmed.ncbi.nlm.nih.gov/26541605
- Polireddy K, Dong R, Reed G, et al. (2017) High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Sci Rep 7: 17188 https://pubmed.ncbi.nlm.nih.gov/29215048
- Alexander MS, Wilkes JG, Schroeder SR, Buettner GR. (2018) Pharmacologic ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Cancer Res. 78:6838-6851. https://pubmed.ncbi.nlm.nih.gov/30254147.
- Drisko JA, Serrano OK, Spruce LR, Chen Q, Levine M. (2018) Treatment of pancreatic cancer with intravenous vitamin C: a case report. Anti-Cancer Drugs 29:373-379. https://pubmed.ncbi.nlm.nih.gov/29438178
- Welsh JL, Wagner BA, van't Erve TJ, et al. (2013) Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 71:765-775. https://pubmed.ncbi.nlm.nih.gov/23381814
- Monti DA, Mitchell E, Bazzan AJ, et al. (2012) Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS ONE 7(1):e29794. https://pubmed.ncbi.nlm.nih.gov/22272248
- Mikirova N, Casciari J, Hunninghake R. (2019) Continuous Intravenous Vitamin C in the Cancer Treatment: Reevaluation of a Phase I Clinical Study. Functional Foods in Health and Disease. https://riordanclinic.org/journal-article-archive/continuous-intravenous-vitamin-c-in-the-cancer-treatment-reevaluation-of-a-phase-i-clinical-study
- Di Tano M, Raucci F, Vernieri C, et al. (2020) Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat Comm 11:2332 https://pubmed.ncbi.nlm.nih.gov/32393788
- Carr AC, Cook J. (2018) Intravenous Vitamin C for Cancer Therapy -- Identifying the Current Gaps in Our Knowledge. Front. Physiol. 9:1182. https://pubmed.ncbi.nlm.nih.gov/30190680
- Cullen JJ (2021) A Phase II Trial of Pharmacological Ascorbate, Gemcitabine, and Nab-Paclitaxel for Metastatic Pancreatic Cancer (PACMAN 2.1) https://clinicaltrials.gov/ct2/show/NCT02905578
- Shah MA, Khan U. (2021) A Phase II Study of High Dose Vitamin C Intravenous Infusion in Patients with Resectable or Metastatic Solid Tumor Malignancies. https://jcto.weill.cornell.edu/open_clinical_trials/a-phase-ii-study-of-high-dose-vitamin-c-intravenous-infusion-in-patients-with-resectable-or-metastatic-solid-tumor-malignancies
- Riordan Clinic (2021) High Dose IV Vitamin C (IVC) https://riordanclinic.org/what-we-do/high-dose-iv-vitamin-c
- Grimble RF. (2001) Nutritional modulation of immune function. Proc. Nutr. Soc. 60:389-397. https://pubmed.ncbi.nlm.nih.gov/11681814
- Arthur JR, McKenzie RC, Beckett GJ. (2003) Selenium in the immune system. J. Nutr. 133:1457S-1459S. https://pubmed.ncbi.nlm.nih.gov/12730442
- Guillin OM, Vindry C, Ohlmann T, Chavatte L. (2019) Selenium, Selenoproteins, and Viral Infection. Nutrients, 11:2101. https://pubmed.ncbi.nlm.nih.gov/31487871
- Huang Z, Rose AH, Hoffman PR. (2012) The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal. 16:705-743. https://pubmed.ncbi.nlm.nih.gov/21955027
- Enqvist M, Nilsonne G, Hammarfjord O, et al, (2011) Selenite Induces Posttranscriptional Blockade of HLA-E Expression and Sensitizes Tumor Cells to CD94/NKG2A-Positive NK Cells. J Immunol. 187:3546-3554. https://pubmed.ncbi.nlm.nih.gov/21890659
- Hiraoka N, Ino Y, Hori S, et al. (2020) Expression of classical human leukocyte antigen class I antigens, HLA-E and HLA-G, is adversely prognostic in pancreatic cancer patients Cancer Sci. 111:3057-3070. https://pubmed.ncbi.nlm.nih.gov/32495519
- Cao S, Durrani FA, Tóth K, Rustum YM. (2014) Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models Br J Cancer 110:1733-1743. https://pubmed.ncbi.nlm.nih.gov/24619073
- Knox SJ, Jayachandran P, Keeling CA, et al. (2019) Results from a Phase 1 Study of Sodium Selenite in Combination with Palliative Radiation Therapy in Patients with Metastatic Cancer. Transl Oncol. 12:1525-1531. https://pubmed.ncbi.nlm.nih.gov/31454725
- Doello K, Mesas C, Quiñonero F, et al. (2021) The Antitumor Activity of Sodium Selenite Alone and in Combination with Gemcitabine in Pancreatic Cancer: An In Vitro and In Vivo Study. Cancers. 13:3169. https://pubmed.ncbi.nlm.nih.gov/34201986
- Husain K, Francois RA, Yamauchi T, et al. (2011) Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther. 10:2363-2372. https://pubmed.ncbi.nlm.nih.gov/21971120
- Malafa MP, Sebti S, (2014) Delta-Tocotrienol Treatment and Prevention of Pancreatic Cancer. Lee Moffitt Cancer Center & Research Institute, University of South Florida (Tampa): US Patent US 8,846,653. https://patentimages.storage.googleapis.com/c7/ff/ef/b836e04b18be57/US8846653.pdf
- Hussein D, Mo H. (2009) d-Delta-tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas. 38:e124-e136. https://pubmed.ncbi.nlm.nih.gov/19346993
- Husain K, Centeno BA, Chen D-T, et al. (2013) Vitamin E delta-tocotrienol prolongs survival in the LSLKrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila). 6:1074-83. https://pubmed.ncbi.nlm.nih.gov/23963802
- Husain K, Centeno BA, Coppola D, et al. (2017) d-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget. 8:31554-31567. https://pubmed.ncbi.nlm.nih.gov/28404939
- Springett GM, Husain K, Neuger A, et al. (2015) A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E d-tocotrienol in Patients with Pancreatic Ductal Neoplasia EBioMedicine 2:1987-1995. https://pubmed.ncbi.nlm.nih.gov/26844278
- Guthrie N, Gapor A, Chambers AF, Carroll KK. (1997) Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr. 127:544S-548S. https://pubmed.ncbi.nlm.nih.gov/9082043
- Nesaretnam K, Stephen R, Dils R, Darbre P. (1998) Tocotrienols inhibit the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids. 33:461-469. https://pubmed.ncbi.nlm.nih.gov/9625593
- Shun M-C, Yu W, Gapor A, et al. (2004) Pro-apoptotic mechanisms of action of a novel vitamin E analog (alpha-TEA) and a naturally occurring form of vitamin E (delta-tocotrienol) in MDA-MB-435 human breast cancer cells. Nutr Cancer. 48:95-105. https://pubmed.ncbi.nlm.nih.gov/15203383
- Kaneko S, Sato C, Shiozawa N, et al. (2018) Suppressive Effect of Delta-Tocotrienol on Hypoxia Adaptation of Prostate Cancer Stem-like Cells. Anticancer Res. 38:1391-1399. https://pubmed.ncbi.nlm.nih.gov/15203383
- Ji X, Wang Z, Geamanu A, et al. (2012) Delta-tocotrienol suppresses Notch-1 pathway by upregulating miR-34a in nonsmall cell lung cancer cells. Int J Cancer. 131: 2668-2677. https://pubmed.ncbi.nlm.nih.gov/22438124
- Ji X, Wang Z, Sarkar FH, Gupta SV. (2012) Delta-tocotrienol augments cisplatin-induced suppression of non-small cell lung cancer cells via inhibition of the Notch-1 pathway. Anticancer Res. 32:2647-2655. https://pubmed.ncbi.nlm.nih.gov/22753722
- Nasr M, Nafee N, Saad H, Kazem A. (2014) Improved antitumor activity and reduced cardiotoxicity of epirubicin using hepatocyte-targeted nanoparticles combined with tocotrienols against hepatocellular carcinoma in mice. Eur J Pharm Biopharm. 88:216-225. https://pubmed.ncbi.nlm.nih.gov/24813390
- Wada S, Naito Y, Matsushita Y, et al. (2017) Delta-tocotrienol suppresses tumorigenesis by inducing apoptosis and blocking the COX-2/PGE2 pathway that stimulates tumor-stromal interactions in colon cancer. J Funct Foods. 35:428-435. https://www.sciencedirect.com/science/article/pii/S1756464617303183
- Shibata A, Nakagawa K, Tsuduki T, Miyazawa T. (2015) Delta-Tocotrienol treatment is more effective against hypoxic tumor cells than normoxic cells: potential implications for cancer therapy. J Nutr Biochem. 26:832-840. https://pubmed.ncbi.nlm.nih.gov/25979648
- Zhang J-S, Li D-M, He N, et al. (2011) A paraptosis-like cell death induced by delta-tocotrienol in human colon carcinoma SW620 cells is associated with the suppression of the Wnt signaling pathway. Toxicology. 285:8-17. https://pubmed.ncbi.nlm.nih.gov/21453743
- Sun W, Wang Q, Chen B, et al. (2008) Gamma-tocotrienol-induced apoptosis in human gastric cancer SGC-7901 cells is associated with a suppression in mitogen-activated protein kinase signalling. Br J Nutr. 99:1247-1254. https://pubmed.ncbi.nlm.nih.gov/18081943
- Sun W, Xu W, Liu H, et al. (2009) gamma-Tocotrienol induces mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J Nutr Biochem. 20:276-284. https://pubmed.ncbi.nlm.nih.gov/18602811
- Satyamitra MM, Kulkarni S, Ghosh SP, et al. (2011) Hematopoietic Recovery and Amelioration of Radiation-Induced Lethality by the Vitamin E Isoform delta-Tocotrienol. Radiat Res. 175:736-745. https://pubmed.ncbi.nlm.nih.gov/21434782
- Constantinou C, Charalambous C, Kanakis D. (2020) Vitamin E and cancer: an update on the emerging role of gamma and delta tocotrienols. Eur J Nutr. 59:845-857. https://pubmed.ncbi.nlm.nih.gov/31016386
- Drotleff AM, Bohnsack C, Schneider I, et al. (2014) Human oral bioavailability and pharmacokinetics of tocotrienols from tocotrienol-rich (tocopherol-low) barley oil and palm oil formulations. J Funct Foods. 7:150-160. https://www.sciencedirect.com/science/article/pii/S1756464614000024
- Szewczyk K, Chojnacka A, Górnicka M. (2021) Tocopherols and Tocotrienols -- Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int J Mol Sci. 22:6222. https://pubmed.ncbi.nlm.nih.gov/34207571
- Trias AM, Tan B. (2013) Alpha-tocopherol: a detriment to tocotrienol benefits. In: Tan B, Watson RR, Preedy VR, eds. Tocotrienols: Vitamin E Beyond Tocopherols. 2nd ed. Boca Raton, FL: CRC Press; 2013. pp 61-78. ISBN-13: 978-1138199729
- Qureshi AA, Pearce BC, Nor RM, et al. (1996) Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr. 126:389-394. https://pubmed.ncbi.nlm.nih.gov/8632210
- Shibata A, Kawakami Y, Kimura T, et al. (2016) Alpha-tocopherol attenuates the triglyceride- and cholesterol-lowering effects of rice bran tocotrienol in rats fed a Western diet. J Agric Food Chem. 64:5361-5366. https://pubmed.ncbi.nlm.nih.gov/27295311
- Shibata A, Nakagawa K, Tsuduki T, Miyazawa T. (2015) Alpha-tocopherol suppresses antiangiogenic effect of delta-tocotrienol in human umbilical vein endothelial cells. J Nutr Biochem, 26:345-50. https://pubmed.ncbi.nlm.nih.gov/25662730
- Passwater R (2019) More Than Vitamin E: The Story & Science Behind Tocotrienols. Whole Foods Magazine. https://wholefoodsmagazine.com/columns/vitamin-connection/more-than-vitamin-e-the-story-science-behind-tocotrienols-part-1-tocotrienols-no-longer-the-obscure-member-of-the-vitamin-e-family
- Penberthy WT, Saul AW, Smith RG, (2021) Niacin and Cancer How vitamin B-3 protects and even helps repair your DNA. Orthomolecular Medicine News Service. http://orthomolecular.org/resources/omns/v17n05.shtml
- Diener E,Chan MY. (2011) Happy People Live Longer: Subjective Well-Being Contributes to Health and Longevity. Applied Psychology: Health and Well-Being. 3:1-43. https://doi.org/10.1111/j.1758-0854.2010.01045.x
- Yuen RCF (2021) High Dose Vitamin C for Cancer: The Struggle with "Non-Evidence-Based" Medical Practice. Orthomolecular Medicine News Service, http://orthomolecular.org/resources/omns/v17n20.shtml
Nutritional Medicine is Orthomolecular Medicine
Orthomolecular medicine uses safe, effective nutritional therapy to fight illness. For more information: http://www.orthomolecular.org
Orthomolecular Medicine News Service (OMNS)
OMNS free subscription link http://orthomolecular.org/subscribe.html
OMNS archive link http://orthomolecular.org/resources/omns/index.shtml
Find a Doctor
To locate an orthomolecular physician near you: http://orthomolecular.org/resources/omns/v06n09.shtml
The peer-reviewed Orthomolecular Medicine News Service is a non-profit and non-commercial informational resource.
Editorial Review Board:
Albert G. B. Amoa, MB.Ch.B, Ph.D. (Ghana)
Seth Ayettey, M.B., Ch.B., Ph.D. (Ghana)
Ilyès Baghli, M.D. (Algeria)
Ian Brighthope, MBBS, FACNEM (Australia)
Gilbert Henri Crussol, D.M.D. (Spain)
Carolyn Dean, M.D., N.D. (USA)
Ian Dettman, Ph.D. (Australia)
Damien Downing, M.B.B.S., M.R.S.B. (United Kingdom)
Susan R. Downs, M.D., M.P.H. (USA)
Ron Ehrlich, B.D.S. (Australia)
Hugo Galindo, M.D. (Colombia)
Martin P. Gallagher, M.D., D.C. (USA)
Michael J. Gonzalez, N.M.D., D.Sc., Ph.D. (Puerto Rico)
William B. Grant, Ph.D. (USA)
Claus Hancke, MD, FACAM (Denmark)
Tonya S. Heyman, M.D. (USA)
Suzanne Humphries, M.D. (USA)
Ron Hunninghake, M.D. (USA)
Bo H. Jonsson, M.D., Ph.D. (Sweden)
Dwight Kalita, Ph.D. (USA)
Felix I. D. Konotey-Ahulu, MD, FRCP, DTMH (Ghana)
Jeffrey J. Kotulski, D.O. (USA)
Peter H. Lauda, M.D. (Austria)
Alan Lien, Ph.D. (Taiwan)
Homer Lim, M.D. (Philippines)
Stuart Lindsey, Pharm.D. (USA)
Victor A. Marcial-Vega, M.D. (Puerto Rico)
Charles C. Mary, Jr., M.D. (USA)
Mignonne Mary, M.D. (USA)
Jun Matsuyama, M.D., Ph.D. (Japan)
Joseph Mercola, D.O. (USA)
Jorge R. Miranda-Massari, Pharm.D. (Puerto Rico)
Karin Munsterhjelm-Ahumada, M.D. (Finland)
Tahar Naili, M.D. (Algeria)
W. Todd Penberthy, Ph.D. (USA)
Zhiyong Peng, M.D. (China)
Isabella Akyinbah Quakyi, Ph.D. (Ghana)
Selvam Rengasamy, MBBS, FRCOG (Malaysia)
Jeffrey A. Ruterbusch, D.O. (USA)
Gert E. Schuitemaker, Ph.D. (Netherlands)
T.E. Gabriel Stewart, M.B.B.CH. (Ireland)
Thomas L. Taxman, M.D. (USA)
Jagan Nathan Vamanan, M.D. (India)
Garry Vickar, M.D. (USA)
Ken Walker, M.D. (Canada)
Anne Zauderer, D.C. (USA)
Andrew W. Saul, Ph.D. (USA), Editor-In-Chief
Associate Editor: Robert G. Smith, Ph.D. (USA)
Editor, Japanese Edition: Atsuo Yanagisawa, M.D., Ph.D. (Japan)
Editor, Chinese Edition: Richard Cheng, M.D., Ph.D. (USA)
Editor, French Edition: Vladimir Arianoff, M.D. (Belgium)
Editor, Norwegian Edition: Dag Viljen Poleszynski, Ph.D. (Norway)
Editor, Arabic Edition: Moustafa Kamel, R.Ph, P.G.C.M (Egypt)
Editor, Korean Edition: Hyoungjoo Shin, M.D. (South Korea)
Editor, Spanish Edition: Sonia Rita Rial, PhD (Argentina)
Contributing Editor: Thomas E. Levy, M.D., J.D. (USA)
Assistant Editor: Helen Saul Case, M.S. (USA)
Technology Editor: Michael S. Stewart, B.Sc.C.S. (USA)
Associate Technology Editor: Robert C. Kennedy, M.S. (USA)
Legal Consultant: Jason M. Saul, JD (USA)
Comments and Media Contact: drsaul@doctoryourself.com OMNS welcomes but is unable to respond to individual reader emails. Reader comments become the property of OMNS and may or may not be used for publication.
Click here to see a web copy of this news release: https://orthomolecular.acemlna.com/p_v.php?l=1&c=209&m=212&s=c7ae1002d2f579a22c16a1b89c854212
Comments:
-
No Article Comments available