Positive Health Online
Your Country

Letters to the Editor Issue 276
listed in letters to the editor, originally published in issue 276 - February 2022
Oxford University Project Using Artificial Intelligence for Safer CT Imaging of Blood Vessels Receives Heart Research UK Grant
National charity Heart Research UK has granted over £246,000 to Professor Regent Lee and his team at the University of Oxford who have developed a computational method which uses artificial intelligence (AI) to generate ‘contrast enhanced’ CT scans without using contrast dye. The new technology may also have a positive impact on the sustainability of healthcare.
Computerised Tomography (CT or CAT) scans are widely used in all fields of medicine and surgery. Where treatment of a blood vessel is being considered, doctors need a detailed view inside the blood vessel. Blood clots can often be found inside arteries or veins, and the position and shape of the clot, in relation to the blood vessel wall, is important for the planning of treatment, such as the placement of a stent.
On routine CT scans, the human eye cannot detect the presence of abnormalities such as blood clots inside the blood vessel. Therefore, a special dye called a contrast agent, is injected into the patient to generate ‘contrast enhanced’ CT scans. This makes it easier to visualise the blood flowing through the blood vessel and to identify the blood clot.
However, there are several problems with the use of contrast dye. Contrast enhanced CT scans take longer to complete and require more radiation exposure for the patient. Patients usually get some mild reactions to the contrast dye and may be allergic to it, in which case they cannot have contrast enhanced CT scans.
Importantly, the use of contrast dye requires an intravenous injection into the patient’s arm. This can be both uncomfortable and can result in extravasation, when the dye may leak out from the injection site to the tissues under the skin causing skin problems. Contrast dye can sometimes damage the kidneys, which can be particularly problematic with elderly patients whose kidneys are not working at full capacity.
Reducing the use of contrast dye could have a positive impact on the environment and sustainability of healthcare. Radioactive contrast agents are a major component of pharmaceutical waste in hospital water systems. They are biologically inert and can linger in the wastewater system for a long time. Although the long-term impact on health is yet to be defined, several European countries are actively seeking solutions to minimise wastewater contamination by radioactive contrast agents.
Professor Lee’s team has developed a technology to generate contrast enhanced CT scans without the need to inject contrast dye. This research uses Al which is an advanced computing technology whereby a computer is programmed to mimic the learning and problem-solving abilities of the human mind. The grant from Heart Research UK will support the team to further develop their technology.
Although human eyes cannot tell the difference between blood flow and clot within the blood vessel on a routine CT scan, there are minute details in the scan that can be used to differentiate them.
Professor Lee said: “The use of artificial intelligence in generating CT scans without the need for contrast dyes would allow diagnosis and treatment of blood vessel conditions with a reduced risk of complications. Furthermore, the reduced scanning time, radiation dose and lower cost would bring important benefits, not limited to the scanning of blood vessels. This method may be used for diagnosis and treatment planning for other medical conditions where contrast enhanced CT scans are required.”
Kate Bratt-Farrar, Chief Executive of Heart Research UK, said: “We are delighted that the grant will be supporting the work of Professor Lee and his team at the University of Oxford, whose ground-breaking work with AI may help to ultimately make the CT scanning of blood vessels safer, without the need for contrast dyes.
“Heart Research UK’s Novel and Emerging Technologies research grants are all about helping patients. We are confident that Professor Lee’s pioneering project will not only utilise the latest and most innovative developments but also benefit those who need them, as soon as possible.”
Heart Research UK
Proud to stand out from the crowd, Heart Research UK is the charity dedicated to your heart. They inspire and invest in pioneering medical research, ground-breaking training and education, and in communities to improve their heart health for themselves. For over 50 years they have driven advancements in the prevention, treatment and cure of heart disease to benefit patients as soon as possible.
In the last 10 years, Heart Research UK has funded over £10.2m in medical research in hospitals and universities across the UK, as well as £2.2m on innovative community-based lifestyle projects to improve the heart health of the nation.
They like a personal approach, so if they want to contact you they do it themselves, and certainly don’t pay anyone to do it. They treat people how they would like to be treated themselves.
If you’d like to support Heart Research UK’s vital work into the prevention, treatment and cure of heart disease, please visit www.heartresearch.org.uk for inspiration on how you could help. Find out more or apply for a grant at Heart Research UK’s Research Grants.
Source and Further Contact
For media enquiries contact Ebba Ritzen, PR and Media Officer at Heart Research UK, via Tel: 0113 234 7474; press@heartresearch.org.uk
Research Reveals How Epithelial Cells in the Body Naturally Eliminate “Precancerous” Ones
Waseda University researchers demonstrate how healthy epithelial cells recognize precancerous ones and the mechanism behind their eventual elimination
Normal epithelial cells show the ability to push out precancerous ones present in the epithelium, by means of “cell competition.” But the exact molecular mechanism of this recognition by normal epithelial cells was unknown. Now, researchers from Waseda University, Japan, have unraveled the interactions and cellular pathways leading to this extrusion, allowing them to identify a candidate for a therapeutic target for future cancer prevention research.
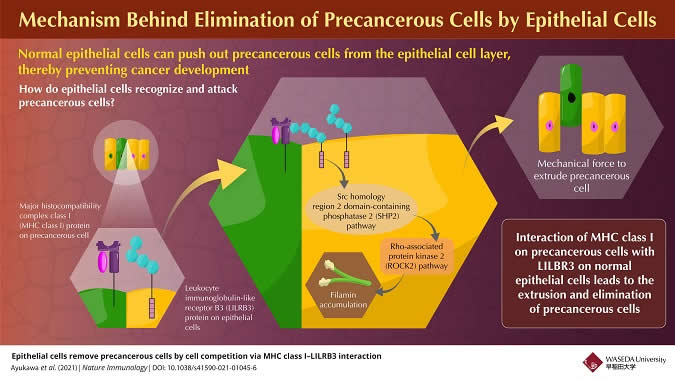
Mechanism Behind Elimination Of Precancerous Cells By Epithelial Cells
Infographic Credit: Waseda University
In addition to its immune surveillance system, recent reports have shown that the human body has defence mechanisms run by non-immune epithelial cells. Epithelial cells are a type of cell that occur in layers that line most surfaces of our body. These epithelial cells can recognize and extrude neighboring precancerous cells from the epithelium; this extrusion process is called cell competition. This form of immune-like surveillance has garnered attention in recent years based on its potential for future immune-like therapeutic targets for cancer preventive treatment. However, it is still unknown what kind of ligand-receptor interactions are involved in the recognition of precancerous cells by normal epithelial cells. Recently, a group of scientists have successfully solved this research question and have published their findings in Nature Immunology.
Speaking about the premise of their study, Professor Takeshi Maruyama, an Associate Professor at the Waseda Institute for Advanced Study at Waseda University, who led the research group, says,
“During the process of cell competition, normal epithelial cells can be primed by contact with precancerous cells. However, it was previously unclear how neighboring normal epithelial cells recognize precancerous cells to eliminate them.”
In this work, the researchers identified a plasma membrane protein, Canis suboptimal alteration recognizing protein (Canis AltR) in non-transformed canine epithelial cells, the function of which was unknown, as a recognizing protein for cell competition. In humans, the protein most similar to AltR is leukocyte immunoglobulin-like receptor B3 (LILRB3). AltR/LILRB3 interacts with major histocompatibility complex class I (MHC class I) that is expressed on precancerous epithelial cells.
MHC class I-AltR/LILRB3 interaction causes the activation of AltR/LILRB3, which triggers an intracellular SHP2–ROCK2 (Src homology-2 domain-containing protein tyrosine phosphatase-2–Rho-associated protein kinase 2) pathway. This SHP2–ROCK2 pathway leads to the “accumulation of cytoskeletal components,” which generates a mechanical force to extrude precancerous cells, in the normal epithelial cells at the boundary with precancerous cells. Finally, normal epithelial cells push the precancerous cells out of the epithelium to eliminate them from the body.
Interestingly, this molecular mechanism occurs independently of natural killer or CD8+ T cell-mediated immune responses.
“Our study describes a new immune-like mechanism by non-immune epithelial cells to suppress tumorigenesis,” says Maruyama.
The researchers hope that these significant findings can be applied to real life cancer treatment.
Maruyama adds, “The recombinant MHC-I-α3 protein used in this study enhances the elimination of precancerous cells and suppresses the formation of tumors and precancerous lesions. We hope that this biomolecule would contribute to a therapeutic candidate for cancer prevention by the elimination of precancerous cells.”
This study advances us closer to more effective cancer treatments in the future, bringing hope to the fight against this devastating disease.
Reference
Shiyu Ayukawa (1), Nagisa Kamoshita (2), Jun Nakayama (1), Ryohei Teramoto (1), Novalia Pishesha (3), Kenji Ohba (4), Nanami Sato (5), Kei Kozawa (5), Hikari Abe (1), Kentaro Semba (1), Nobuhito Goda (1), Yasuyuki Fujita (5,6), Takeshi Maruyama (2). Epithelial cells remove precancerous cells by cell competition via MHC class I–LILRB3 interaction. Journal: Nature Immunology DOI: https://doi.org/10.1038/s41590-021-01045-6
Affiliations:
- School of Advanced Science and Engineering, Waseda University, Japan
- Waseda Institute for Advanced Study, Waseda University, Japan
- Faculty of Arts and Sciences, Harvard University, USA
- Jichi Medical University, Japan
- Hokkaido University Graduate School of Chemical Sciences and Engineering, Japan
- Kyoto University Graduate School of Medicine, Japan
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university that has long been dedicated to academic excellence, innovative research, and civic engagement at both the local and global levels since 1882. The University ranks number one in Japan in international activities, including the number of international students, with the broadest range of degree programs fully taught in English. To learn more about Waseda University, visit https://www.waseda.jp/top/en
Source and Further Contact
Brijesh Manek <brijesh.manek@cactusglobal.com>
Vitamin C and Cortisol: Synergistic Infection and Toxin Defense
Commentary by Thomas E Levy MD JD
Republished from orthomolecular.activehosted.com
Vitamin C and cortisol are the two most important and most powerful naturally-occurring anti-inflammatory agents. The mechanisms of their synergistic action indicate they are literally designed by nature to interact together to optimize the antioxidant impact needed to resolve the disease-causing oxidation that always results from toxins, infections, and stress. As inflammation in a tissue is the direct result of the oxidation, metabolism, and depletion of vitamin C levels in that tissue, it is of primary concern to normalize cellular vitamin C levels as promptly and completely as possible. Quite literally, when intracellular vitamin C levels are normalized in an inflamed tissue, the inflammation is completely resolved, and the cells are once again in a non-diseased, normal state.
A focal vitamin C depletion in a tissue is the primary identifying feature of focal inflammation. Greater degrees of focal inflammation only occur with greater degrees of focal oxidative stress. As can logically be expected, focal oxidative stress rises as focal vitamin C stores are consumed and are not restored. This lack of vitamin C at sites of inflammation explains nicely why the acute immune system response to a focally inflamed tissue is initially dominated by the appearance of monocytes (Tabas et al., 2017). The monocytes have an exceptionally high concentration of vitamin C in them, the highest of all the immune cells. Relative to the plasma concentration of vitamin C, these monocytes concentrate vitamin C in their cytoplasm 80-fold (8,000%) higher than the plasma. Other immune cells also have very high intracellular levels of vitamin C (Evans et al., 1982). It appears likely that the initial role of monocytes arriving at a site of inflammation is to effectively deliver antioxidant impact in the form of vitamin C, working to promptly alleviate whatever degree of oxidative stress is present.
In many hospitalized patients with significant infections, extremely low plasma levels of vitamin C are present. When the depressed levels of vitamin C are present throughout the body and not focal, the associated increased oxidative stress is generalized and typically reflected in elevated blood levels of C-reactive protein (CRP). CRP is a reliable index of systemically increased inflammation that is always present when vitamin C levels are significantly low (Carr et al., 2017). Circulating cortisol levels are also the lowest in the most severely ill patients.
As it turns out, cortisol significantly augments the uptake of vitamin C into cells (Fujita et al., 2001; Mikirova et al., 2019). More specifically, it appears to stimulate the production of the messenger RNA needed to increase the expression of the sodium-ascorbate co-transporters (SVCTs). This works to enhance cellular vitamin C uptake needed to maximize the protection of metabolically active cells against oxidative stress (Savini et al., 2008). This is very likely the primary function of cortisol in the body, as there is nothing more important for the resolution of tissue inflammation and the resulting tissue damage than normalizing elevated levels of intracellular oxidative stress as rapidly and completely as possible by normalizing intracellular levels of vitamin C. And when intracellular levels of vitamin C are normal, cellular glutathione levels needed to protect the cell are also optimized.
General Disease Physiology
The physiology of all disease at the cellular and biomolecular level relates directly to the extent to which any of a variety of biomolecules are in the oxidized (electron-depleted) state. All pro-oxidants (toxins) ultimately inflict their damage by directly oxidizing biomolecules, or by indirectly resulting in the oxidation of those biomolecules (proteins, sugars, fats, enzymes, etc.). When biomolecules become oxidized (lose electrons) they no longer perform their normal chemical or metabolic functions. An oxidized enzyme, for example, can be completely inactive.
No toxin can cause any clinical toxicity unless biomolecules end up becoming oxidized. The unique array of biomolecules that become oxidized determines the nature of the clinical condition resulting from a given toxin exposure. There is no "disease" present in cells of the tissue involved in a given medical condition beyond the distribution of, identity of, and degree of oxidation in the biomolecules of an affected tissue. Rather than "causing" disease, the state of oxidation in an array of biomolecules IS the disease.
When antioxidants can donate electrons and restore a normal electron status back to previously oxidized biomolecules (reduction), the normal functions of these biomolecules are restored. This is the reason why sufficient antioxidant therapy, such as can be achieved by highly-dosed intravenous vitamin C, has proven to be so profoundly effective in blocking and even reversing the negative clinical impact of any toxin or poison. There exists no toxin against which vitamin C has been tested that has not been effectively neutralized (Levy, 2002).
Because of this, there is no better way to save a patient clinically poisoned by any agent than by immediately administering a sizeable intravenous infusion of sodium ascorbate. The addition of magnesium chloride to the infusion is also important to protect against sudden life-threatening arrhythmias that can occur before a sufficient number of the newly-oxidized biomolecules can be reduced and any remaining toxin is neutralized and excreted (Levy, 2019). The relationship between cortisol and vitamin C also mandates the addition of cortisol to such vitamin C infusions to optimize the rapidity and degree to which poisoned cells can normalize intracellular vitamin C. This directly and promptly reverses the abnormal increases of intracellular oxidation seen with any excessive toxin (poison or pro-oxidant) exposure.
As a practical point, then, the primary clinical point to take from the synergism of vitamin C and cortisol is the following:
Whenever cortisol is clinically indicated, its impact will be greatly enhanced by the simultaneous administration of vitamin C.
AND
Whenever vitamin C is clinically indicated, its impact will be greatly enhanced by the simultaneous administration of cortisol.
Cortisol Physiology
Cortisol, referred to as hydrocortisone when given as a medication, is a hormone known as a glucocorticoid. This type of hormone is produced in the outer portion (cortex) of the adrenal glands that sit on top of the kidneys. In addition to having a pronounced anti-inflammatory effect, a glucocorticoid increases glucose levels in the blood through a process known as gluconeogenesis in the liver. This process utilizes amino acids and other non-carbohydrate molecules to produce more glucose. When cortisol or other corticosteroids are too highly-dosed and given for too long a period of time, a state of widespread protein breakdown (catabolism with muscle wasting) can result as the proteins are converted to glucose. Furthermore, this continued stimulation of glucose production in the liver can result in higher circulating glucose levels and sometimes even frank diabetes. These effects account for some of the most significant side effects of chronic and highly-dosed steroid (e.g., prednisone, dexamethasone) therapy. "Traditionally-dosed" long-term steroid therapy would never cause problems if much lower doses were employed (20 mg of hydrocortisone or less daily), especially when given in conjunction with multigram doses of vitamin C. The chronic ingestion of high doses of steroids without the simultaneous intake (or internal production) of vitamin C is analogous to trying to shoot a high-powered gun without ammunition.
Of note, taking large enough amounts of vitamin C supplementation alone can eliminate the need for more cortisol to optimize intracellular uptake of the vitamin C. However, it is often not a practical option to administer the 50-, 75-, or 100-gram infusions needed to reach such optimal cellular vitamin C levels without the assistance of cortisol. Nevertheless, cortisol still greatly facilitates this process, and having enough cortisol in the bloodstream decreases the "wasting" of vitamin C by its elimination in the kidneys that would otherwise end up inside the cells.
Supplying the right amount of cortisol when it is chronically deficient and no longer being synthesized in normal amounts in the body is still absolutely essential to achieving optimal health, similar to the need for thyroid hormone administration when its levels are chronically low.
Even though the body might have "normal" levels of cortisol upon blood testing at different times in the day, this does not rule out that under conditions of severe stress and new infection/toxin exposure the adrenals might no longer have the capacity to produce sufficient additional amounts of cortisol to deal with that stress. In fact, succumbing to an infection is a direct indication that more cortisol (and vitamin C) was needed by the body. It has been observed that a fatigued individual with known adrenal insufficiency can readily progress to an influenza-like state of malaise and generalized aching when the cortisol level is especially low. Patients with clear-cut influenza have markedly low cortisol levels, and the lowest cortisol levels occur in the sickest patients with the highest fevers and the lowest white counts. Any acute severe infection results in the same classical symptoms associated with just very low cortisol levels, as seen in patients with acute adrenal insufficiency (Jefferies, 2004).
The potent anti-inflammatory effect of cortisol fits perfectly with its label as the anti-stress, "fight-or-flight" hormone. Physiologically, stress is effectively a surge of pro-oxidants (toxins) into the blood, whether from infection or another source. This results in a need for the body to immediately counteract or compensate with a surge of antioxidants. In a completely normal mammalian liver, vitamin C is synthesized from glucose modified by a sequence of four enzymes. However, most humans are missing the fourth enzyme due to an epigenetic defect.
Part of the "fight-or-flight" reaction to stress in the body is also supported by the release of adrenaline (epinephrine) from the inner part (medulla) of the adrenal glands. Adrenaline works to mobilize glucose from its storage form (glycogen) in the liver and muscles, and it also stimulates gluconeogenesis to further increase glucose levels (Cryer, 1993). This would appear to be important in making sure that enough glucose is available to the fully-functioning liver to make whatever amount of vitamin C is needed to deal with a severe enough acute infection or toxin insult. Of note, vitamin C supplementation has been shown to decrease circulating cortisol and adrenaline levels in athletes following stressful exercise. This is consistent with the role played by these two substances to increase vitamin C levels following any form of stress. When enough vitamin C is already present, cortisol and adrenaline are no longer as necessary in supporting the response of the body to stress, and their levels are appropriately lower (Peters et al., 2001).
Nevertheless, the natural design of this anti-stress, antitoxin effect in the body is incredibly elegant when vitamin C synthesis can occur in a completely normal liver, as is the case with many mammals. It can be summarized as follows:
- The presence of pro-oxidant pathogens or other toxins ("stress") in the blood results in
- A compensatory increased liver production of vitamin C released directly into the blood to neutralize the toxin surge, along with an accompanying reflex release of cortisol and adrenaline from the adrenal glands, which results in
- An increased uptake of the newly-synthesized vitamin C into the toxin-exposed cells by the increased presence of the cortisol in the blood, which is sustained by
- A cortisol-induced increased glucose production (gluconeogenesis) in the liver and an adrenaline-induced release of glucose from its storage forms (glycogen) which results in
- An ongoing conversion of that increased glucose production into more vitamin C production with an ongoing release of cortisol to bring the vitamin C inside the toxin-challenged cells, continuing until
- The infection is resolved and/or the toxins are fully neutralized with electrons, metabolized, and excreted.
However, in the typical human who is missing the fourth enzyme in the liver needed to synthesize more vitamin C from glucose, the cortisol has only the pre-existing vitamin C circulating in the blood available for cellular uptake. At the same time, the cortisol-induced and adrenaline-induced production of more glucose will chronically contribute to its excess presence throughout the body since it cannot be used to fuel the production of more vitamin C in the liver. Of note, a recently-discovered olive-derived polyphenol appropriately-dosed appears to help overcome this epigenetic defect, or at least to boost systemic levels of vitamin C in the body [www.formula216.com]. Regular supplementation with this product appears to be very effective in optimizing vitamin C impact in the body.
When the acute oxidative stress is due to the onset of infection and not solely due to the presence of a new toxin in the blood, the cortisol also plays an important role in killing the pathogen. By facilitating vitamin C entry into the infected cell, cortisol serves to help upregulate the Fenton reaction. This reaction utilizes the electrons supplied by the cellular vitamin C to break down the cytoplasmic hydrogen peroxide into the highly lethal hydroxyl radical, which immediately oxidizes every biomolecule it encounters, ultimately resulting in pathogen death, programmed cell death (apoptosis), and/or frank cellular rupture (Levy, 2021).
Supporting Research
The nature of this important interplay between vitamin C and cortisol is further supported and clarified by a substantial amount of clinical, animal, and basic (in vitro) research data.
- In human lung microvascular endothelial cells, vitamin C and hydrocortisone work to synergistically and dramatically reverse lipopolysaccharide-induced (oxidative) barrier dysfunction (Barabutis et al., 2017).
- In a rat model of kidney reperfusion injury, vitamins C and E in combination with hydrocortisone appear to offer synergistic protection compared to that offered by the individual agents (Azari et al., 2015).
- Hydrocortisone function, or expression, depends on the redox status of its intracellular receptor. When a substantial percentage of the receptors are oxidized, the degree of hydrocortisone binding to its receptors is proportionately lessened, and hydrocortisone can no longer optimize vitamin C uptake into the cell (Okamoto et al., 1999).
- A vitamin C derivative restored electrons to oxidized hydrocortisone receptors, which allowed them to function (Okamoto et al., 1998). Such inactivated receptors are increased in number in the highly oxidized environment of infection. This means that sufficient vitamin C is needed to keep receptors activated and able to bind whatever hydrocortisone is present inside the cell, which then can further facilitate the uptake of more vitamin C. A classical synergism: more cellular vitamin C leads to more cortisol receptor binding, and more cortisol receptor binding leads to more cellular vitamin C uptake.
- In patients with asthma, vitamin C supplementation has been shown to permit a reduction in the corticosteroid dose required to maintain control of that condition, further supporting the similar physiological impacts of vitamin C and hydrocortisone (Fogarty et al., 2006).
The degree of infection (as with mild influenza versus advanced sepsis) largely determines whether the administration of hydrocortisone will be of significant additional benefit in the treatment protocol. Advanced sepsis is a condition in which systemic oxidative stress is about as maximal as it can be before proceeding to death. As such, a very substantial percentage of the intracellular hydrocortisone receptors are in a nonfunctional, oxidized state. Because of this, the body attempts to compensate by increasing the production of cortisol in the body, although this offers little to no benefit as long as the receptors remain oxidized and unable to bind any cortisol, and no vitamin C is being administered to activate the receptors.
In less advanced infections, and even in earliest stages of sepsis, the number of receptors is often increased and administering hydrocortisone can have clear-cut benefits, especially when vitamin C is given as well (Vardas et al., 2017). In fact, increased receptor function is essential to prevent a person or laboratory animal with early sepsis from proceeding to advanced sepsis and death. As the infection advances, receptor function is depressed due to the increased oxidation of a worsening infection, and the body then transitions to the increased production of cortisol in an attempt to compensate (Antonucci et al., 2014; Shibata et al., 2015). This almost never stops the clinical decline, and death ensues unless enough vitamin C is given to activate the oxidized receptors and significantly decrease overall oxidative stress by increasing intracellular levels of vitamin C. Similar findings have been seen in an animal model of sepsis (Bergquist et al., 2013).
The treatment of patients in septic shock with vitamin C, hydrocortisone, and thiamine was reported to be stunningly effective, with the mortality rate dropping from 40% to 9%, and with none of the deaths resulting directly from sepsis or septic complications (Marik et al., 2017). However, a similarly constructed study showed that basically the same result could be achieved with only the administration of vitamin C (Zabet et al., 2016). This fits with the observation that circulating endogenous levels of cortisol are already elevated in advanced sepsis, and it is vitamin C administration, not additional hydrocortisone, that is of most consequence at that point in the treatment of a septic patient. Of note, as primary or secondary therapy, vitamin C has attenuated sepsis-induced adult respiratory distress syndrome (Bharara et al., 2016), aspiration-induced adult respiratory distress syndrome (Kim et al., 2017), virus-induced adult respiratory distress syndrome (Fowler et al., 2017), and adult respiratory distress syndrome secondary to the complications of pustular psoriasis (Marik and Long, 2018).
The best therapy for any advanced sepsis patient would simply be very large doses of vitamin C intravenously, on the order of 25 grams every six hours (100 grams every 24 hours). Along with the pre-existing high circulating levels of cortisol, this would rapidly reduce elevated intracellular oxidative stress levels to normal or near-normal, and all but those sepsis patients who had already developed too much multi-organ damage would be readily saved.
On the other hand, many critically ill patients who are not fighting sepsis will demonstrate significantly low cortisol levels (Marik et al., 2008), and they would benefit greatly from the administration of both vitamin C and hydrocortisone. Also, whenever there is a question of whether cortisol levels are already high in the body, the addition of further hydrocortisone does no harm and can readily be added to the protocol to "cover all bases."
Overall, the current scientific literature indicates that vitamin C and hydrocortisone individually promote increased antioxidant capacity. However, it is also clear that these two agents are very synergistic in promoting this effect, although properly-dosed vitamin C also appears to be very effective as a monotherapy in sepsis and septic shock.
Vitamin C and Cortisol Safety
Before proceeding to the recommended applications of a combined therapeutic approach with vitamin C and hydrocortisone, the current state of propaganda directed at undermining and limiting the use of these agents should be addressed. Most physicians have been completely misled into believing that vitamin C is toxic to the kidneys and promotes the formation of kidney stones. Nothing could be further from the truth. As with all the other organs in the body, vitamin C, in multigram daily doses, only promotes good health throughout the body, including the kidneys. A Harvard study on 85,557 women with no history of kidney stones showed that a regular intake of vitamin C was not associated with any risk of developing kidney stones (Curhan et al., 1999). Another Harvard study actually found that individuals with the highest vitamin C intake had a lower risk of kidney stones than those individuals with the lowest vitamin C intake (Gerster, 1997). This was further corroborated in another study looking at blood levels of vitamin C in over 10,000 subjects. The subjects with the highest blood levels had the lowest incidence of kidney stones (Simon and Hudes, 1999).
Highly-dosed vitamin C given intravenously also causes no problems with kidney function and does not promote the formation of kidney stones. Such infusions achieve temporary blood levels much higher than with oral administration, yet still are completely nontoxic. A prospective study following 157 patients given such infusions showed no kidney problems developing over a period of 12 months. No stones were reported, even though 8% of the patients already had a history of kidney stones (Prier et al., 2018). Vitamin C, along with magnesium, vitamin D, and vitamin K2 all work to prevent stone formation as well as to dissolve and mobilize existing stones. This is because stones are usually calcium oxalate, and the oxalate that can come from vitamin C metabolism will never produce a stone in the absence of an excessive calcium presence (Levy, 2013). In fact, even though it is chemically a weak organic acid, vitamin C (ascorbic acid) puts calcium carbonate into solution as readily as a concentrated inorganic acid, like hydrochloric acid (Ruskin, 1938).
Aside from the myth of vitamin C causing kidney stones, there are many physicians who just seem to think it must be toxic and would not even considering giving it intravenously. In fact, vitamin C might be the only substance for which a toxic level cannot be established. Continuous vitamin C infusions of 50 grams daily were given over an eight-week period in advanced cancer patients with no definable negative side effects (Casciari et al., 2001). A study surveying the administration of infusions routinely exceeding 25 grams in over 20,000 patients cared for by 172 complementary medicine practitioners revealed the infusion to be "remarkably safe" (Padayatty et al., 2010). At the Riordan Clinic in Wichita, KS over a 16-year period "...194,054 g, or 427 lbs of IV vitamin C" was administered to 275 patients with no significant side effects ever observed (Jackson et al., 2002). For even further perspective on this remarkable lack of toxicity by vitamin C, consider the fact that too much water ingested too rapidly can kill (Hayashi et al., 2005).
With regard to cortisol, all doctors and most of the public know that high doses of corticosteroids given for an extended period of time have severe and inevitable negative side effects. This has caused the more routine applications of much lower doses of cortisol to also be approached with unnecessary caution. In fact, very many people have abnormally low circulating levels of cortisol. And even more importantly, the degree of stress-induced cortisol release can be significantly decreased even when the non-stressed circulating levels are within the range considered to be normal. If most people were routinely tested for their circulating cortisol levels and the degree to which they are able to increase cortisol release in response to stress, the routine administration of cortisol in daily doses of 20 mg or less would be commonly employed in acute infections as well as in the long-term treatment of chronic medical conditions (Jefferies, 2004).
Optimizing the Treatment of Spike Protein Persistence
While adding hydrocortisone to the administration of vitamin C can further improve an already excellent therapy, the use of this combined therapy appears to be an optimal way to approach syndromes that are characterized by persistence of the COVID-related spike protein in the body. Individuals experiencing problems following COVID vaccinations, as well as "long-haul" COVID, which is basically a low-grade and ongoing chronic COVID infection, should prove to be optimal candidates for treatment protocols that include combined hydrocortisone and vitamin C administration. As mentioned above, vitamin C alone given in sufficient doses can still effectively "saturate" the targeted cells, but the doses required simply make many physicians with only limited vitamin C experience too uncomfortable to give such doses, whereas the same result can be achieved with lesser doses of vitamin C combined with hydrocortisone.
The thorough and complete treatment of persistent spike protein is especially important for not only reducing long-term mortality but also for reducing a great deal of morbidity, or clinical illness, in the shorter term. While it now appears that an ongoing spike protein persistence can result in a very wide array of clinical syndromes, depending what organs or tissues most bind the spike protein in different individuals, many appear to maintain inflammation in the heart muscle. A substantial number of such patients appear to have a low-grade, smouldering myocarditis that will eventually evolve to cardiac "burnout" and a fatal congestive cardiomyopathy. For additional therapeutic guidance for these patients, see this article: [http://orthomolecular.org/resources/omns/v17n24.shtml]. This myocarditis (heart muscle inflammation) can manifest as fatigue, intermittent chest pain, shortness of breath, abnormal heart rhythms, and sometimes even the development of inflammation and blood clotting problems in the coronary arteries that can lead to heart attacks. It is vital that this inflammation gets vigorously treated and completely resolved. As such, there should be a high index of suspicion of its presence in anyone with even minimal symptoms after having had a COVID infection, as well as in anyone who has had a COVID vaccination, which involves the direct administration of the spike protein. Simply assume the spike protein is present and replicating, and proceed with an aggressive protocol to eliminate it completely.
Many viruses and pathogens, especially COVID, will typically persist in the body, especially in the upper and lower gastrointestinal tract. Because of this, anyone who feels completely recovered from COVID but who never received a definitive virus-killing treatment in the course of recovery (ivermectin, ozone, vitamin C, hydrogen peroxide nebulization, etc.) would be well-advised to follow the recommendations in the article noted above. Totally asymptomatic individuals who had blood microscopy examinations weeks after COVID vaccinations showed striking evidence of pathological red blood cell stickiness. This alone is clear justification for the application of vitamin C (with hydrocortisone if possible) along with any of a number of the other bio-oxidative therapies to resolve this stickiness as completely as possible. Of note, hydrogen peroxide nebulization is especially important in eliminating persistent pathogen presence anywhere in the gastrointestinal tract, which is the "pathogen reservoir" that most permits the persistence of COVID or any other pathogen following clinical resolution of the acute infection (Levy, 2021).
General Guide for the Administration of Hydrocortisone with Vitamin C
As is discussed at great length elsewhere, the importance of vitamin C in cellular physiology combined with the epigenetic defect in the liver preventing its synthesis in the body mandate that multi-gram daily doses of vitamin C should be part of the supplementation regimen of everyone (Levy, 2002). Optimal health can never be achieved and maintained on the miniscule RDA of 75 to 90 mg of vitamin C per day for women and men. Optimal daily intake is much closer to amounts that are in excess of 100-fold greater than these RDA recommendations. Furthermore, the amounts of vitamin C needed during periods of advanced oxidative stress can be in excess of 1000-fold more than the RDA amounts. For an extensive guide to the multifaceted administration of vitamin C, see: Thomas-Levy-Guide-To-The-Optimal-Administration-of-Vitamin-C.pdf
While a clinical goal of normalizing health and returning abnormal laboratory tests to normal can often be achieved with many of the different approaches to vitamin C supplementation as outlined in the Guide above, there are also a number of clinical circumstances that do not readily normalize and would benefit greatly from the addition of hydrocortisone to optimize intracellular vitamin C levels. Furthermore, the appropriate addition of hydrocortisone to a vitamin C treatment protocol at the outset saves otherwise wasted steps in optimizing intracellular health as quickly as possible. Any of the recommendations described below should be administered and followed with a qualified health care practitioner. These recommendations are in addition to whatever else is being recommended in a treatment protocol, whether for an acute or a chronic condition.
For Acute Infections
When Intravenous Vitamin C Is an Option
For 25 to 50 grams vitamin C infusions, 50 mg of hydrocortisone can be added to each infusion (or given as an IV push after infusion is started); lesser amounts of vitamin C (7.5 to 25 grams as an infusion or even as an IV push) can still be given with a total of 25 to 50 mg of hydrocortisone in the syringes as well [Riordan-Clinic-IVC-Push-Protocol]. If only oral hydrocortisone is available, 20 mg should be given orally approximately one hour before the vitamin C infusion or IV push is administered. This timing synchronizes the peak blood levels of the hydrocortisone and vitamin C. This approach can be continued until the acute infection (usually one to two weeks or less) is resolved.
When Intravenous Vitamin C is not an Option
5 grams of oral liposome-encapsulated vitamin C [www.livonlabs.com], along with 4 to 6 grams of sodium ascorbate powder (heaping teaspoon) in water or juice. This can be repeated several times daily based on clinical response. Other forms of oral vitamin C can be similarly-dosed. One vitamin C administration can be accompanied with 5 to 15 mg of hydrocortisone orally. It is best not to exceed a cumulative daily dose of 15 mg of hydrocortisone if this oral option is intended to be continued indefinitely.
For Chronic Infections and Chronic Diseases
It is optimal for patients in this group to receive testing to determine baseline and stress-related blood levels of cortisol. This establishes clearly the underlying adequacy of the adrenal glands for producing cortisol under both baseline and circumstances of acute oxidative stress. While everybody can benefit from the vitamin C-hydrocortisone combinations being presented, this testing can better identify those patients who most need this kind of antioxidant support indefinitely. Optimizing intracellular vitamin C must be a lifelong therapeutic goal.
As caring for patients with chronic infections and chronic diseases is highly individualized, there can be no fixed recommendations. Availability, convenience, and expense are important dictating factors in how often someone can receive vitamin C infusions. When this is an early part of a long-term treatment protocol, the recommendations noted for acute infections can be employed, and after a couple weeks, the oral vitamin C/hydrocortisone approach can be adopted. When vitamin C infusions are given intermittently but indefinitely, as one or more times monthly for a cancer patient, the hydrocortisone can always be added.
Many patients can benefit from simply taking 5 mg of hydrocortisone orally every time they take their oral form of vitamin C, up to three times daily (15 mg of hydrocortisone total per day). However, all of these possibilities can only be realized with the guidance of a physician or other health care professional who is closely following the clinical response and serial blood testing of a given patient, and who is able to prescribe the oral hydrocortisone tablets. The potential variations in the application of vitamin C with hydrocortisone are numerous.
Summary
Hydrocortisone plays an active role in facilitating the uptake of vitamin C into the cells of the body. Since the ultimate health of any cell is directly reflected in the vitamin C status in the cytoplasm, attention should always be paid to taking whatever measures are available for optimizing the concentrations of vitamin C in all the cells of the body. Furthermore, both vitamin C and hydrocortisone have been established to be the most potent and naturally available anti-inflammatory agents in existence. It would appear that the ability of hydrocortisone to augment cellular vitamin C uptake is likely the primary reason that it has its potent anti-inflammatory properties.
While very highly-dosed vitamin C does not require "assistance" to optimize its intracellular levels, relatively few physicians are comfortable applying such dosages. Because of this, combining hydrocortisone along with lower-dosed vitamin C can greatly increase the number of patients who can still optimize their health with vitamin C therapy.
OMNS Contributing Editor Dr Thomas E. Levy is board certified in internal medicine and cardiology. He is also an attorney, admitted to the bar in Colorado and in the District of Columbia. The views presented in this article are the author's and not necessarily those of all members of the Orthomolecular Medicine News Service Editorial Review Board.
References
Antonucci E, Fiaccadori E, Taccone F, Vincent J (2014) Glucocorticoid administration in sepsis and septic shock: time for a paradigm change? Minerva Anestesiologica 80:1058-1062. PMID: 24971687
Azari O, Kheirandish R, Azizi S et al (2015) Protective effects of hydrocortisone, vitamin C and E alone or in combination against renal ischemia-reperfusion injury in rat. Iranian Journal of Pathology 10:272-280. PMID: 26351497
Barabutis N, Khangoora V, Marik P, Catravas J (2017) Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest 152:954-962. PMID: 28739448
Bergquist M, Nurkkala M, Rylander C et al. (2013) Expression of the glucocorticoid receptor is decreased in experimental Staphylococcus aureus sepsis. The Journal of Infection 67:574-583. PMID: 23933016
Bharara A, Grossman C, Grinnan D et al. (2016) Intravenous vitamin C administered as adjunctive therapy for recurrent acute respiratory distress syndrome. Case Reports in Critical Care 2016:8560871. PMID: 27891260
Carr A, Rosengrave P, Bayer S et al. (2017) Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Critical Care 21:300. PMID: 29228951
Casciari J, Riordan N, Schmidt T et al. (2001) Cytotoxicity of ascorbate, lipoic acid, and other antioxidants in hollow fibre in vitro tumours. British Journal of Cancer 84:1544-1550. PMID: 11384106
Cryer P (1993) Adrenaline: a physiological metabolic regulatory hormone in humans? International Journal of Obesity and Related Metabolic Disorders 17 Suppl 3:S43-S46. PMID: 8124400
Curhan G, Willett W, Speizer F, Stampfer M (1999) Intake of vitamins B6 and C and the risk of kidney stones in women. Journal of the American Society of Nephrology 10:840-845. PMID: 10203369
Evans R, Currie L, Campbell A (1982) The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. The British Journal of Nutrition 47:473-482. PMID: 7082619
Fogarty A, Lewis S, Scrivener S et al. (2006) Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respiratory Medicine 100:174-179. PMID: 16338599
Fowler A, Kim C, Lepler L et al. (2017) Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World Journal of Critical Care Medicine 6:85-90. PMID: 28224112
Fujita I, Hirano J, Itoh N et al. (2001) Dexamethasone induces sodium-dependant vitamin C transporter in a mouse osteoblastic cell line MC3T3-E1. The British Journal of Nutrition 86:145-149. PMID: 11502226
Gerster H (1997) No contribution of ascorbic acid to renal calcium stones. Annals of Nutrition & Metabolism 41:269-282. PMID: 9429689
Hayashi T, Ishida Y, Miyashita T et al. (2005) Fatal water intoxication in a schizophrenic patient-an autopsy case. Journal of Clinical Forensic Medicine 12:157-159. PMID: 15914312
Jackson J, Riordan H, Bramhall N, Neathery S (2002) Sixteen-year history with high dose intravenous vitamin C treatment for various types of cancer and other diseases. Journal of Orthomolecular Medicine 17:117-119.
Jefferies W (2004) Safe Uses of Cortisol. Springfield, Illinois: Charles C Thomas Publisher
Kim C, Debesa O, Nicolato P et al. (2017) Vitamin C infusion for gastric acid aspiration-induced acute respiratory distress syndrome (ARDS). Pulmonary Research and Respiratory Medicine Open Journal 4:33-37.
Levy T (2002) Curing the Incurable. Vitamin C, Infectious Diseases, and Toxins. Henderson, NV: MedFox Publishing
Levy T (2013) Death by Calcium: Proof of the toxic effects of dairy and calcium supplements. Henderson, NV: MedFox Publishing
Levy T (2019) Magnesium, Reversing Disease. Henderson, NV: MedFox Publishing
Levy T (2021) Rapid Virus Recovery: No need to live in fear! Henderson, NV: MedFox Publishing. Free eBook download (English or Spanish) available at https://rvr.medfoxpub.com
Marik P, Pastores S, Annane D et al. (2008) Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical Care Medicine 36:1937-1949. PMID: 18496365
Marik P, Khangoora V, Rivera R et al. (2017) Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock. Chest 151:1229-1238. PMID: 27940189
Marik P, Long A (2018) ARDS complicating pustular psoriasis: treatment with low-dose corticosteroids, vitamin C and thiamine. BMJ Case Reports 2018. PMID: 29420246
Mikirova N, Levy T, Hunninghake R (2019) The levels of ascorbic acid in blood and mononuclear blood cells after oral liposome-encapsulated and oral non-encapsulated vitamin C supplementation, taken without and with IV hydrocortisone. Journal of Orthomolecular Medicine 34:1-8.
Okamoto K, Tanaka H, Makino Y, Makino I (1998) Restoration of the glucocorticoid receptor function by the phosphodiester compound of vitamins C and E, EPC-K1 (L-ascorbic acid 2-[3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-yl hydrogen phosphate] potassium salt), via a redox-dependent mechanism. Biochemical Pharmacology 56:79-86. PMID: 9698091
Okamoto K, Tanaka H, Ogawa H et al. (1999) Redox-dependent regulation of nuclear import of the glucocorticoid receptor. The Journal of Biological Chemistry 274:10363-10371. PMID: 10187825
Padayatty S, Sun A, Chen Q et al. (2010) Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One 5:e11414. PMID: 20628650
Peters E, Anderson R, Nieman D et al. (2001) Vitamin C supplementation attenuates the increases in circulating cortisol, adrenaline and anti-inflammatory polypeptides following ultramarathon running. International Journal of Sports Medicine 22:537-543. PMID: 11590482
Prier M, Carr A, Baillie N (2018) No reported renal stones with intravenous vitamin C administration: a prospective case series study. Antioxidants 7:68. PMID: 29883396
Ruskin S (1938) Studies on the parallel action of vitamin C and calcium. The American Journal of Digestive Diseases 5:408-411.
Savini I, Rossi A, Pierro C et al. (2008) SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34:347-355. PMID: 17541511
Shibata A, Troster E, Wong H(2015) Glucocorticoid receptor expression in peripheral WBCs of critically ill children. Pediatric Critical Care Medicine 16:e132-e140. PMID: 25850866
Simon J, Hudes E (1999) Relation of serum ascorbic acid to serum vitamin B12, serum ferritin, and kidney stones in US adults. Archives of Internal Medicine 159:619-624. PMID: 10090119
Tabas I, Lichtman A (2017) Monocyte-macrophages and T cells in atherosclerosis. Immunity 47:621-634. PMID: 29045897
Vardas K, Ilia S, Sertedaki A et al. (2017) Increased glucocorticoid receptor expression in sepsis is related to heat shock proteins, cytokines, and cortisol and is associated with increased mortality. Intensive Care Medicine Experimental 5:10. PMID: 28224564
Zabet M, Mohammadi M, Ramezani M, Khalili H (2016) Effect of high-dose ascorbic acid on vasopressor's requirement in septic shock. Journal of Research in Pharmacy Practice 5:94-100. PMID: 27162802
Nutritional Medicine is Orthomolecular Medicine
Orthomolecular medicine uses safe, effective nutritional therapy to fight illness. For more information: http://www.orthomolecular.org
Orthomolecular Medicine News Service (OMNS)
OMNS free subscription link http://orthomolecular.org/subscribe.html
OMNS archive link http://orthomolecular.org/resources/omns/index.shtml
Find a Doctor
To locate an orthomolecular physician near you: http://orthomolecular.org/resources/omns/v06n09.shtml
The peer-reviewed Orthomolecular Medicine News Service is a non-profit and non-commercial informational resource.
Editorial Review Board
Albert G. B. Amoa, MB.Ch.B, Ph.D. (Ghana)
Seth Ayettey, M.B., Ch.B., Ph.D. (Ghana)
Ilyès Baghli, M.D. (Algeria)
Ian Brighthope, MBBS, FACNEM (Australia)
Gilbert Henri Crussol, D.M.D. (Spain)
Carolyn Dean, M.D., N.D. (USA)
Ian Dettman, Ph.D. (Australia)
Damien Downing, M.B.B.S., M.R.S.B. (United Kingdom)
Susan R. Downs, M.D., M.P.H. (USA)
Ron Ehrlich, B.D.S. (Australia)
Hugo Galindo, M.D. (Colombia)
Martin P. Gallagher, M.D., D.C. (USA)
Michael J. Gonzalez, N.M.D., D.Sc., Ph.D. (Puerto Rico)
William B. Grant, Ph.D. (USA)
Claus Hancke, MD, FACAM (Denmark)
Tonya S. Heyman, M.D. (USA)
Suzanne Humphries, M.D. (USA)
Ron Hunninghake, M.D. (USA)
Bo H. Jonsson, M.D., Ph.D. (Sweden)
Dwight Kalita, Ph.D. (USA)
Felix I. D. Konotey-Ahulu, MD, FRCP, DTMH (Ghana)
Jeffrey J. Kotulski, D.O. (USA)
Peter H. Lauda, M.D. (Austria)
Alan Lien, Ph.D. (Taiwan)
Homer Lim, M.D. (Philippines)
Stuart Lindsey, Pharm.D. (USA)
Victor A. Marcial-Vega, M.D. (Puerto Rico)
Charles C. Mary, Jr., M.D. (USA)
Mignonne Mary, M.D. (USA)
Jun Matsuyama, M.D., Ph.D. (Japan)
Joseph Mercola, D.O. (USA)
Jorge R. Miranda-Massari, Pharm.D. (Puerto Rico)
Karin Munsterhjelm-Ahumada, M.D. (Finland)
Tahar Naili, M.D. (Algeria)
W. Todd Penberthy, Ph.D. (USA)
Zhiyong Peng, M.D. (China)
Isabella Akyinbah Quakyi, Ph.D. (Ghana)
Selvam Rengasamy, MBBS, FRCOG (Malaysia)
Jeffrey A. Ruterbusch, D.O. (USA)
Gert E. Schuitemaker, Ph.D. (Netherlands)
T.E. Gabriel Stewart, M.B.B.CH. (Ireland)
Thomas L. Taxman, M.D. (USA)
Jagan Nathan Vamanan, M.D. (India)
Garry Vickar, M.D. (USA)
Ken Walker, M.D. (Canada)
Anne Zauderer, D.C. (USA)
Andrew W. Saul, Ph.D. (USA), Editor-In-Chief
Associate Editor: Robert G. Smith, Ph.D. (USA)
Editor, Japanese Edition: Atsuo Yanagisawa, M.D., Ph.D. (Japan)
Editor, Chinese Edition: Richard Cheng, M.D., Ph.D. (USA)
Editor, French Edition: Vladimir Arianoff, M.D. (Belgium)
Editor, Norwegian Edition: Dag Viljen Poleszynski, Ph.D. (Norway)
Editor, Arabic Edition: Moustafa Kamel, R.Ph, P.G.C.M (Egypt)
Editor, Korean Edition: Hyoungjoo Shin, M.D. (South Korea)
Editor, Spanish Edition: Sonia Rita Rial, PhD (Argentina)
Contributing Editor: Thomas E. Levy, M.D., J.D. (USA)
Assistant Editor: Helen Saul Case, M.S. (USA)
Technology Editor: Michael S. Stewart, B.Sc.C.S. (USA)
Associate Technology Editor: Robert C. Kennedy, M.S. (USA)
Legal Consultant: Jason M. Saul, JD (USA)
Comments and Media Contact
drsaul@doctoryourself.com OMNS welcomes but is unable to respond to individual reader emails. Reader comments become the property of OMNS and may or may not be used for publication.
Acknowledgement Citation
Republished from orthomolecular.activehosted.com
Being Overweight may Cause More UK Hospital Admissions than Previously Thought
Being overweight may cause more hospital admissions and higher incidences of disease and mortality than previous studies report, according to new University of Bristol-led research. The study, published in Economics and Human Biology,[1] used a genetic technique to identify the sole impact of body composition on hospital admissions from over 300,000 people. The study, led by researchers from Bristol Medical School’s Population Health Sciences, aimed to find out the impact of excess body fat on the yearly hospital admission rate in the UK by analyzing body mass index (BMI) data – a marker of overall body fat – and waist-hip ratio (WHR) data – a marker of regional body fat – from 310,471 individuals within the UK Biobank cohort. Linked with this data was information on 550,000 UK inpatient hospital admissions, with participants followed up for an average of six years.
Using this data, researchers compared estimates from conventional epidemiological analyses with a method called Mendelian randomisation, a genetic technique which allows scientists to quantify how being overweight may be causally related to disease and mortality. The method uses genetic changes in the genome linked to body composition to estimate the causal effect of being overweight on a health outcome and remove the effects of other factors that may jointly influence body composition and rates of hospital admission.
Their results found evidence for a direct causal effect of higher BMI and WHR on higher yearly hospital admission rates, with estimates that were larger than those obtained from existing research. One of the team’s most striking discoveries showed the relationship was largely driven by an adverse fat distribution in a certain area (measured by waist-hip ratio) rather than overall BMI. They found that people were between 16 per cent to 26 per cent more likely to be admitted to hospital with each 0.09-unit higher waist-hip ratio compared to 8 per cent to 16 per cent with each 4.74kg/m2 higher BMI. For example, for a woman of average height (163 cm) and weight (65kg) in this study, and average measurements of waist (86 cm) and hip (103 cm) in this study, this would be the equivalent of gaining 9.3 cm (or 3.7 inches) in waist circumference, and just under 13kg in weight, respectively. For a man of average height (177 cm) and weight (79 kg) in this study, and average measurements of waist (94 cm) and hip (102 cm) in this study, this would correspond to a 9.2 cm (or 3.6 inches) increase in waist circumference and a 15kg increase in weight.
Dea Hazewinkel, the study’s lead researcher from the University’s Bristol Medical School and Population Health Sciences Institute, said:
“We live in increasingly obesogenic environments with the World Health Organisation identifying 39 per cent of men and 40 per cent of women as being overweight, and 11 per cent of men and 15 per cent of women as obese worldwide. Finding causal effect estimates between fatty tissue and hospital admissions larger than those previously reported in existing studies emphasises the necessity of exploring policies aimed at reducing obesity in the population.
“The results also suggest that a preference should be given to waist-hip ratio as a measure of body fat over BMI as this may be more important for predicting hospital admissions.”
The study was funded through grants from the Medical Research Council, the Elizabeth Blackwell Institute for Health Research, and The Wellcome Trust.
Reference
- Audinga-Dea Hazewinkel, Rebecca C.Richmond, Kaitlin H.Wade and Padraig Dixon. Mendelian randomization analysis of the causal impact of body mass index and waist-hip ratio on rates of hospital admission. Economics and Human Biology: 44: January 2022.
About the Medical Research Council
The Medical Research Council is at the forefront of scientific discovery to improve human health. Founded in 1913 to tackle tuberculosis, the MRC now invests taxpayers’ money in some of the best medical research in the world across every area of health. Thirty-three MRC-funded researchers have won Nobel prizes in a wide range of disciplines, and MRC scientists have been behind such diverse discoveries as vitamins, the structure of DNA and the link between smoking and cancer, as well as achievements such as pioneering the use of randomised controlled trials, the invention of MRI scanning, and the development of a group of antibodies used in the making of some of the most successful drugs ever developed. Today, MRC-funded scientists tackle some of the greatest health problems facing humanity in the 21st century, from the rising tide of chronic diseases associated with ageing to the threats posed by rapidly mutating micro-organisms. The Medical Research Council is part of UK Research and Innovation. https://mrc.ukri.org/
About Wellcome
Wellcome supports science to solve the urgent health challenges facing everyone. We support discovery research into life, health and wellbeing, and we’re taking on three worldwide health challenges: mental health, global heating and infectious diseases.
About the Elizabeth Blackwell Institute for Health Research
Nurturing research. Improving health.
The Elizabeth Blackwell Institute drives innovation in research to improve health for all. It nurtures interdisciplinary research to address the complex health challenges facing us today
The institute focuses on:
- Supporting the next generation of health researchers
- Connecting people to develop interdisciplinary research
- Including everyone in research so the research can benefit all.
Further Information and Contact
For further information or to arrange an interview with the researchers please contact Joanne Fryer [Mon to Wed], Mobile: +44 (0)7747 768805; joanne.fryer@bristol.ac.uk, or Caroline Clancy [Wed to Fri], Mobile: +44 (0)7776 170238; caroline.clancy@bristol.ac.uk, at the University of Bristol Press Office.
Comments:
-
No Article Comments available